News
A new state-by-state analysis shows a statistical association between high adherence to mask wearing and reduced rates of COVID-19 in the U.S. Charlie Fischer and colleagues at the Boston University School of Public Health in Massachusetts present these findings in the open-access journal PLOS ONE on [DATE].
During the COVID-19 pandemic, different states have enacted different policies on mask wearing, with some states having no mask requirements and others requiring masks in all public spaces. Understanding the link between mask wearing and COVID-19 rates could help inform policies to mitigate stress on healthcare systems, economic instability, and death.
To help clarify the effects of mask wearing, Fischer and colleagues examined publicly available data on mask-wearing policies, people’s self-reported habits on mask wearing in public, and COVID-19 rates for all 50 U.S. states and Washington, D.C. They accounted for a one-month delay between mask wearing and its subsequent potential impact on COVID-19 rates from May through October 2020. For this analysis, they considered rates of more than 200 cases per 100,000 residents to be high.
The analysis showed that, out of 15 states that did not require people to wear masks in public, 14 had high COVID-19 rates. Meanwhile, eight states had self-reported adherence rates of 75 percent of greater, and none of these states had a high COVID-19 rate. States with the lowest adherence rates had the greatest likelihood of high COVID-19 rates in the subsequent month.
The eight states with at least 75-percent adherence to mask wearing had a mean COVID-19 rate of 109.26 per 100,000 residents in the subsequent month, while the mean COVID-19 rate was 239.99 for states with less than 75 percent adherence.
These findings provide new evidence in support of mask-wearing as a major factor that contributes to reduced COVID-19 rates. They suggest that policies and public health efforts to reduce the spread of COVID-19 should include a focus on improved mask adherence throughout the U.S.
Chicago, Ill. (March 25, 2021) – The Alden Network announced the promotion of Cyeria Brown, BSN, RN to Regional Nurse Consultant. As a new graduate in 2014, Brown was hired as a staff nurse at Alden Debes Rehabilitation and Health Care Center in Rockford, Illinois. Because of her integrity, passion for patient safety and her dedication to clinical excellence, she was promoted to Assistant Director of Nursing in 2017 and in less than a year was promoted to Director of Nursing. In her new role as Regional Nurse Consultant, Brown will focus on regulatory compliance, clinical outcomes and the provision of care, programs and services and will identify opportunities to improve patient care and safety throughout her assigned region of post-acute and rehabilitation facilities. Brown obtained her associate of applied science degree from Kishwaukee College and later obtained her bachelor’s degree in nursing from Chamberlain College of Nursing where she is currently pursuing her master’s degree. Additionally, she is licensed by the State of Illinois as a Registered Nurse and is a certified Infection Preventionist. For more than 50 years, The Alden Network has provided health care and residential solutions for seniors and has helped them function to the best of their ability and live life as independently as possible. To learn more about The Alden Network, please call 1-800-291-5900 or visit www.thealdennetwork.com.
Study author and board-certified dermatologist encourages the public to get vaccinated
Newswise — ROSEMONT, Ill. (April 7, 2021) — As COVID-19 vaccination ramps up globally, new research published today in the Journal of the American Academy of Dermatology demonstrates the wide variety of skin rashes, including full-body rashes, observed after COVID-19 vaccination. The authors provide reassurance that these reactions are generally mild, resolve on their own, and should not deter the public from getting vaccinated.
“We understand that some of these reactions may look scary, but when they appear more than four hours after receiving the COVID-19 vaccine, they are typically minor and in some cases, may indicate the body’s immune system is doing a good job of responding to the vaccine,” says senior study author and board-certified dermatologist Esther Freeman, MD, PhD, FAAD, director of Global Health Dermatology at Massachusetts General Hospital and principal investigator of the international COVID-19 Dermatology Registry. “Some rashes may appear a day or two after vaccination, and some have a delayed onset, as long as 7-14 days after vaccination. Most of these rashes resolve on their own with time or — depending on the rash — may require oral antihistamines, topical steroids, or other treatments as directed by a physician.”
Dr. Freeman does note that any reactions that start immediately after vaccination, or within four hours of the shot, need to be taken very seriously, and patients experiencing these rare type of allergic symptoms should seek prompt medical attention, as recommended by the CDC.
Dr. Freeman’s research examined the Moderna and Pfizer vaccines — two of the most widely administered vaccines authorized for emergency use by the FDA — in the U.S. from December 2020 to February 2021. The research of 414 skin reactions logged in the COVID-19 Dermatology Registry from healthcare workers, including board-certified dermatologists, identified a broad range of skin reactions. These include 218 cases of large, delayed reactions near the injection site — dubbed “COVID vaccine arm” — as well as other types of rashes that include rashes at the injection spot, hives, and full-body rashes similar to those typically seen after viral infections.
Dr. Freeman also says that some patients have developed pernio/chilblains, or what has been called “COVID toes”, following COVID-19 vaccines. She notes that this is of particular interest because it shows that the vaccine is triggering a similar immune response as can be seen after the virus. While these reactions are uncomfortable, she says, they are not necessarily a bad thing. It shows that your body is mounting an immune response to the vaccine, she says, which, in some cases, shows up on your skin.
“As dermatologists, we view the skin as a window into what is happening elsewhere in your body,” says Dr. Freeman. “Through this research, we have a deeper understanding of how the COVID-19 vaccine affects our patients and their skin, and I hope our findings, which show that people tolerated vaccination well even when they did develop skin side effects, offer greater reassurance for anyone who is hesitant to get vaccinated.”
In addition to studying a large spectrum of skin reactions to the COVID-19 vaccines, the researchers assessed patients’ responses from the first dose to the second. They found that less than half of the people who experienced skin reactions after the first dose experienced a reaction after the second, and if they did, it was milder.
“I hope this information encourages more people to get their second dose of the COVID-19 vaccine even if they experienced a skin reaction after their first dose,” says Dr. Freeman. “The COVID-19 vaccine will help protect you from getting the virus and can also prevent you from getting very sick if you do get infected.”
If you have concerns about a rash or other skin reaction that develops after getting the COVID-19 vaccine, don’t hesitate to call your doctor or a board-certified dermatologist.
To find a board-certified dermatologist in your area, visit aad.org/findaderm.
Which Senescent Cells Turn On Genes That Encode for Secreted Tumor-regulating Factors
Newswise — PHILADELPHIA — (April 1, 2021) — Scientists at The Wistar Institute identified a new mechanism of transcriptional control of cellular senescence that drives the release of inflammatory molecules that influence tumor development through altering the surrounding microenvironment. The study, published in Nature Cell Biology, reports that methyltransferase-like 3 (METTL3) and 14 (METTL14) proteins moonlight as transcriptional regulators that allow for establishment of the senescence-associated secretory phenotype (SASP).
Cellular senescence is a stable state of growth arrest in which cells stop dividing but remain viable and produce an array of inflammatory and growth-promoting molecules collectively defined as SASP. These molecules account for the complex crosstalk between senescent cells and neighbouring cells and the effect of cellular senescence in various physiological processes and diseases. Although senescence is regarded as a potent barrier for tumor development, the SASP plays a stage-dependent role during tumor development, mediating the clearance of premalignant lesions during intiation and promoting the growth of established tumors.
“Senescent cells undergo widespread changes in gene expression needed to adapt their phenotype and functions,” said Rugang Zhang, Ph.D., deputy director of The Wistar Institute Cancer Center, Christopher M. Davis Professor and leader of the Immunology, Microenvironment & Metastasis Program. “We pointed out a new mechanism that allows cells to turn on a set of genes encoding for the SASP molecules and may potentially be targeted to inhibit this aspect of senescence while preserving its antitumor function.”
Zhang, who is senior author on the study, and his team focused on METTL3 and METTL14, proteins known for chemically modifying messenger RNA to regulate its function. They found a new role of these proteins in senescence and regulation of gene expression that is independent of their RNA-modifying function.
Depleting cells of METTL3 and METTL14, researchers observed reduced expression of SASP genes, such as inflammatory cytokines, but no effect on cell cycle arrest or other markers of senescence, indicating that decrease in SASP is not an indirect consequence of overall senescence inhibition.
“Our results indicate that METTL3 and METTL14 promote expression of SASP genes, in accordance with other studies that revealed an oncogenic role for these two proteins,” said Pingyu Liu, Ph.D., first author of the study and a staff scientist in the Zhang Lab.
The team further analyzed the association of METTL3 and METTL14 with DNA, comparing senescent and control cells. While the two proteins are found together on DNA in control cells, in senescent cells they have different distribution patterns, whereby METTL3 tends to sit upstream of SASP genes, near the transcription start site, while METTL14 binds away from gene bodies, on regulatory elements called enhancers.
Researchers demonstrated that through this positioning pattern and interacting with each other, METTL3 and METTL14 bring closer together two DNA sequences that in non-senescent cells are distant, allowing the formation of promoter-enhancer chromatin loops. As a consequence, expression of the SASP genes is turned on.
“Although we focused on senescence, we envision that the transcription-regulating function of METTL3 and METTL14 may be involved in many other biological processes beyond our current study,” concluded Zhang.
Co-authors: Jianhuang Lin, Takeshi Fukumoto, Timothy Nacarelli, Xue Hao, and Andrew V. Kossenkov from The Wistar Institute; Fuming Li and M. Celeste Simon from University of Pennsylvania.
Work supported by: National Institutes of Health (NIH) grants R01CA160331, R01CA163377, R01CA202919, R01CA239128, R01CA243142, P01AG031862 to R.Z., P50CA228991, and R50CA211199; U.S. Department of Defense grants OC180109 and OC190181. Additional support was provided by The Honorable Tina Brozman Foundation for Ovarian Cancer Research and The Tina Brozman Ovarian Cancer Research Consortium 2.0; and Ovarian Cancer Research Alliance (Collaborative Research Development Grant #596552 and Ann and Sol Schreiber Mentored Investigator Award #649658). Core support for The Wistar Institute was provided by the Cancer Center Support Grant P30CA010815.
Publication information: m6A-independent genome-wide METTL3 and METTL14 redistribution drives senescence-associated secretory phenotype, Nature Cell Biology, 2021. Online publication.
Photo: The Wistar Institute
Wistar's Dr. Rugang Zhang (center) and lab members
Newswise — CHARLOTTESVILLE, Va., March 29, 2021 – An international team of researchers led by a University of Virginia School of Medicine professor is warning that scientists must better prepare for the next pandemic – and has developed a plan to do just that.
Noting the “avalanche” of scientific data generated in response to COVID-19, UVA’s Wladek Minor, PhD, and colleagues are calling for the creation of an “advanced information system” (AIS) to help scientists integrate, monitor and evaluate the vast amounts of data that will be produced as researchers reveal the molecular architecture of the next pathogen posing a big biological threat. This information on the shape, structure and function of a pathogen is essential to the development of medications, vaccines and treatments. For example, the COVID-19 vaccines now available target the “spike” protein on the surface of the SARS-CoV-2 virus.
Their heavily cited online resource for COVID-19 (https://covid-19.bioreproducibility.org/) demonstrates the usefulness of their approach and can be used as a foundation for the new research strategy, they say. The site includes carefully validated 3-D structural models of numerous proteins related to the SARS-CoV-2 virus, including many potential drug targets.
“Structural models and other experimental results produced by various laboratories must follow a standard evaluation procedure to ensure that they are accurate and conform to accepted scientific standards,” said Minor, Harrison Distinguished Professor of Molecular Physiology and Biological Physics at UVa. “Standardized validation is important for all areas of biomedical sciences, especially for structural models, which are often used as a starting point in subsequent research, such as computer-guided drug docking studies and data mining. Even seemingly insignificant errors can lead such research astray.”
Battling a Pandemic
One important role of AIS would be to identify structures that can be refined and improved, the researchers say. They were happy to note that inspection of the molecular blueprints produced for components of COVID-19 and deposited in the Protein Data Bank online database suggests that most were very good. Less than 1% needed significant reinterpretation and less than 10% could be optimized by moderate revisions.
Still, good buildings require good blueprints. The same is true with vaccines and disease treatments. It’s critical, the researchers say, that the structural and other data for pathogens are as accurate as possible, and that scientists from various fields are speaking the same language when discussing and using them. The proposed AIS would help ensure conformity across disciplines.
“Almost 100,000 COVID-19-related papers have been published and over a thousand models of macromolecules encoded by SARS-CoV-2 have been experimentally determined in about a year. No single human can possibly digest this volume of information,” Minor said. “We believe that the most promising solution to information overload and the lack of effective information retrieval is the creation of an advanced information system that is capable of harvesting results from all relevant resources and presenting the information in instructive ways that promote understanding and knowledge.”
The researchers acknowledge that implementing their proposal would be a major undertaking. Other resources that sought to offer similar benefits on a smaller scale have already come and gone. That’s why it’s so important, the scientists say, that we act now. “Creating an AIS will undoubtedly require the collaboration of many scientists who are experts in their respective fields, but it seems to be the only way to prepare biomedical science for the next pandemic,” the researchers write in a new scientific paper outlining their proposal.
“In the history of humanity, the COVID-19 pandemic is relatively mild by comparison with the bubonic plague (Black Death) that killed a hundred times more people,” the researchers conclude. “We might not be so lucky next time.”
New Approach Outlined
The researchers – from UVA, the National Cancer Institute, Poland and Austria – have detailed their plan in an article in the scientific journal IUCrJ. The article is featured on the journal cover. The resarch team consists of Marek Grabowski, Joanna M. Macnar, Marcin Cymborowski, David R. Cooper, Ivan G. Shabalin, Miroslaw Gilski, Dariusz Brzezinski, Marcin Kowiel, Zbigniew Dauter, Bernhard Rupp, Alexander Wlodawer, Mariusz Jaskolski and Minor.
In their paper, the researchers gratefully acknowledged the financial support of the National Institutes of Health’s National Institute of General Medical Sciences, grant R01-GM132595; the Polish National Agency for Academic Exchange, grant PN/BEK/2018/1/00058/U/00001; the Polish National Science Center, grant 2020/01/0/NZ1/00134; the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research; FWF (Austrian Science Foundation), grant P 32821; and the Polish National Science Centre, grant 2018/29/B/ST6/01989.
Minor and his longtime collaborator Zbyszek Otwinowski, PhD, of the University of Texas Southwestern Medical Center, were recently awarded the Tadeusz Sendzimir Applied Sciences Award by the Polish Institute of Arts and Sciences of America for their efforts to develop and promote software for biomedical applications in the structural biology field.
To keep up with the latest medical research news from UVA, subscribe to the Making of Medicine blog at http://makingofmedicine.virginia.edu.
Newswise — In spring 2020, when the first wave of the coronavirus pandemic hit Finland, older adults drastically reduced their out-of-home activities. During the period of government restrictions, physical exercise was the most common reason to leave home, a recent study at the University of Jyväskylä Faculty of Sport and Health Sciences finds.
"In spring 2020, it was feared that the closure of many activity destinations and the recommendations to avoid close contact with persons from other households put in place by the government would decrease physical activity levels, and thus, negatively affect older adults' physical functional capacity," Senior Researcher Erja Portegijs explains. "According to our research results, this was however, not the case."
Throughout the restriction period, physical exercise and walking outdoors, for example, in nature was possible, and even encouraged by the government later in the spring.
"This study shows that physical exercise was the most common reason to go out," Portegijs adds. "Otherwise, older participants had few reasons to go out beyond grocery shopping during the first spring of the pandemic."
Previous research shows that all activities outside of one's home are beneficial for physical activity. As the reasons to leave home were markedly limited during the first spring of the pandemic, more research is needed to determine the long-term effects on mobility and maintaining functional capacity.
"This research is unique, even though it was based on the data of 44 participants only," Portegijs says. "Previously, we did not know where older adults moved and for what reason. Studying where people go to is possible using a map-based questionnaire. This is one of the first studies utilizing such a questionnaire among older adults."
As coronavirus-related measures have varied significantly between countries, it is not sure whether these results are generalizable to other countries. In Finland, curfews were not implemented and governmental restrictions were mostly based on recommendations rather than enforced regulations.
In 2017 and 2018, a map-based questionnaire was used to collect data on frequently visited activity destinations as part of the larger AGNES study among 75-, 80-, and 85-year-old adults living in Jyväskylä city in Central Finland. In May and June 2020, participants were invited to complete the map-based questionnaire following a postal questionnaire. Only a small portion of participants was able to use digital devices independently and thus to participate. These participants had somewhat better health and function than the others.
"As abilities to use digital devices improve among the aging population, the relevance of map-based research methods will further increase," Portegijs reflects.
Photo: University of Jyväskylä
Physical exercise was the most common reason to leave home during the first wave of the pandemic in 2020.
Newswise — PHILADELPHIA—Hormone drugs that reduce androgen levels may help disarm the coronavirus spike protein used to infect cells and stop the progression of severe COVID-19 disease, suggests a new preclinical study from researchers in the Abramson Cancer Center at the University of Pennsylvania and published online in Cell Press’s iScience.
Researchers show how two receptors—known as ACE2 and TMPRSS2—are regulated by the androgen hormone and used by SARS-CoV-2 to gain entry into host cells. Blocking the receptors with the clinically proven inhibitor Camostat and other anti-androgen therapies prevented viral entry and replication, they also showed in lab studies.
The findings provide more insight into the molecular mechanisms of the virus but also support the use of anti-androgen therapies to treat COVID-19 infections, which are currently being investigated in clinical trials and have produced promising results. They also support data showing increased mortality and severity of disease among men compared to women, who have much lower levels of androgen.
“We provide the first evidence that not only TMPRSS2, which is known to be regulated by androgen, but ACE2 can also be directly regulated by this hormone,” said senior author Irfan A. Asangani, PhD, an assistant professor of Cancer Biology in the Perelman School of Medicine at the University of Pennsylvania. “We also show that the SARS-CoV-2 spike relies on these two receptors to impale and enter cells, and that they can be blocked with existing drugs. That’s important because if you stop viral entry, you reduce the viral load and disease progression.”
Camostat is a drug approved for use in Japan to treat pancreatitis that inhibits TMPRSS2. Other anti-androgen therapies, including androgen deprivation therapy used to treat prostate cancer, serve similar functions.
Driven by the disparity in COVID-19 rates between men and women, the cancer researchers sought to better understand the role androgen and its receptors played in infections, which has long been known to be a driver of prostate cancer.
The researchers performed experiments with a pseudotype SARS-CoV-2, which carries the spike proteins of the virus but not its genome.
In mice with significantly reduced androgen levels and cells treated with anti-androgen treatments, the researchers found little to no expression of TMPRSS2 and ACE2, suggesting both are regulated by the hormone. They also observed how inhibiting TMPRSS2 with Camostat blocked priming of the spike for entry into cells. That drug, as well as enzalutamide, an anti-androgen therapy used to treat prostate cancer, also blocked the virus’ entry into lung and prostate cells. Combining these therapies, they found, significantly reduced virus entry into cells.
“Together, our data provide a strong rationale for clinical evaluations of TMPRSS2 inhibitors, androgen-deprivation therapy / androgen receptor antagonists alone or in combination with antiviral drugs as early as clinically possible to prevent COVID-19 progression,” the authors wrote.
In March, researchers from Brazil reported preliminary results of 600 hospitalized patients in a clinical trial investigating proxalutamide, a new anti-androgen therapy, for the treatment of COVID-19. The drug reduced mortality risk by 92 percent and shortened the median hospital stay by nine days versus the standard of care, the researchers reported.
Next, Asangani and his colleagues will partner with Susan R. Weiss, PhD, a professor of Microbiology and co-director of the Penn Center for Research on Coronaviruses and Other Emerging Pathogens, to investigate the findings further using live SARS-CoV-2, as well as anti-androgen therapies’ ability to block different variants of the virus, which continue to emerge and are often differentiated by their spike proteins.
Penn co-authors of the study include Qu Deng, Reyaz ur Rasool, Ronnie M. Russell, and Ramakrishnan Natesan.
The study was supported by the National Institutes of Health (R01 CA249210-0), a Department of Defense Idea Development Award, a Conquer Cancer Now Award, and Sarcoma Foundation of America.
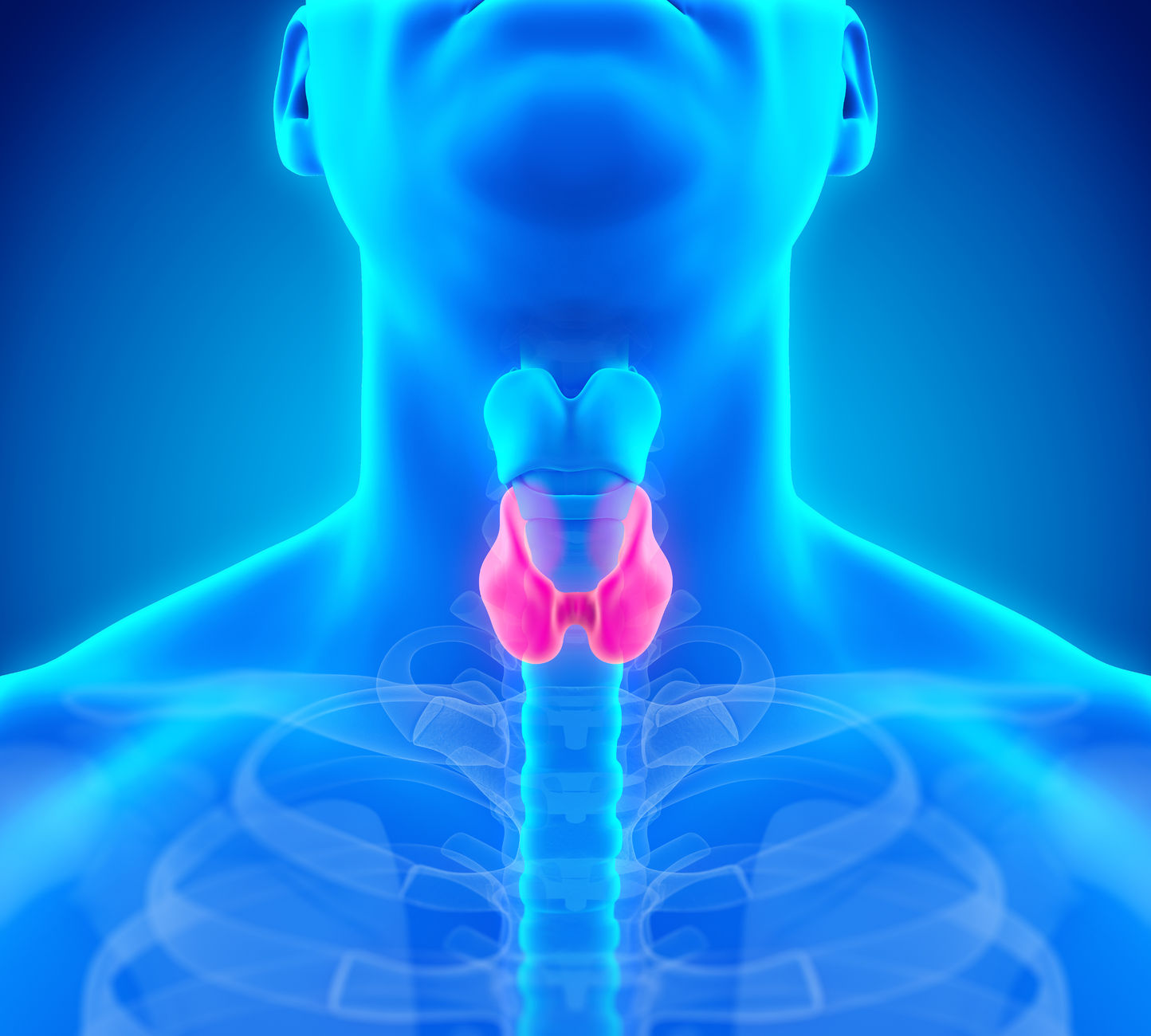
NEARLY A THIRD OF OLDER THYROID PATIENTS TAKE MEDICATIONS THAT INTERFERE WITH THYROID FUNCTION TESTS
Newswise — Nearly one-third of adults age 65 and older who take thyroid hormone also take medications that are known to interfere with thyroid function tests, according to a study presented virtually at ENDO 2021, the Endocrine Society’s annual meeting.“Our findings highlight the complexity of managing thyroid hormone replacement in older adults, many of whom take medications for other medical conditions,” said first author Rachel Beeson, M.D., of the University of Michigan in Ann Arbor, Mich. “Until now, the prevalence of concurrent use of thyroid hormone and interfering medications in older adults, and patient characteristics associated with this practice, has been unknown.”Thyroid hormone use is very common in older adults. Levothyroxine, used to treat hypothyroidism (low thyroid hormone), is one of the most frequently prescribed medications in the United States. Thyroid function tests are used to determine the dose and effectiveness of treatment. The results of these tests can be altered by a variety of medications.Beeson and colleagues analyzed data from 538,137 adults age 65 and older who used thyroid hormone. They looked at how many patients concurrently took thyroid hormone and medications that commonly interfere with thyroid function tests, such as prednisone, prednisolone, carbamazepine, phenytoin, phenobarbital, amiodarone, lithium, interferon-alpha and tamoxifen.Overall, 31.6% of patients were taking medications that have been known to interfere with thyroid function tests.“When we examined patient characteristics associated with concurrent use of thyroid hormone and at least one interfering medication, this was more likely to be seen in patients who were female, non-white and of Hispanic ethnicity,” Beeson said. The researchers also found people who had other chronic medical conditions were more likely to concurrently use thyroid hormone and medications that interfere with thyroid tests.The National Institute on Aging supported the research with a grant to senior author Maria Papaleontiou, M.D.
Researchers at Rutgers School of Dental Medicine have found evidence that two types of mouthwash disrupt the COVID-19 virus under laboratory conditions, preventing it from replicating in a human cell.
The study, published in the journal Pathogens, found that Listerine and the prescription mouthwash Chlorhexidine disrupted the virus within seconds after being diluted to concentrations that would mimic actual use. Further studies are needed to test real-life efficacy in humans.
The study was conducted in a lab using concentrations of the mouthwash and the time it would take to contact tissues to replicate conditions found in the mouth, said Daniel H. Fine, the paper’s senior author and chair of the school’s Department of Oral Biology.
The study found two other mouthwashes showed promise in potentially providing some protection in preventing viral transmission: Betadine, which contains povidone iodine, and Peroxal, which contains hydrogen peroxide. However, only Listerine and Chlorhexidine disrupted the virus with little impact on skin cells inside the mouth that provide a protective barrier against the virus.
“Both Povidone iodine and Peroxal caused significant skin cell death in our studies, while both Listerine and Chlorhexidine had minimal skin cell killing at concentrations that simulated what would be found in daily use,” said Fine.
The team studied the efficacy of mouthwash potential for preventing viral transmission to better understand how dental providers can be protected from aerosols exhaled by patients. “As dentists, we’re right there in a patient’s face. We wanted to know if there’s something that might lower the viral load,’’ said coauthor Eileen Hoskin, an assistant professor at Rutgers School of Dental Medicine.
Fine cautions the public against relying on mouthwash as a way to slow the spread until it is proven in clinical trials on humans.
“The ultimate goal would be to determine whether rinsing two or three times a day with an antiseptic agent with active anti-viral activity would have the potential to reduce the ability to transmit the disease. But this needs to be investigated in a real-world situation,’’ he said.
Previous research has shown various types of antiseptic mouthwashes can disrupt the novel coronavirus and temporarily prevent transmission, but this was one of the first studies that examined antiseptic rinse concentrations, time of contact and the skin-cell killing properties that simulated oral conditions. The study was conducted by a team of dental school scientists and virologist at the Public Health Research Institute.
“Since the SARS CoV-2 virus responsible for COVID-19 enters primarily through the oral and nasal cavity, oral biologists should be included in these studies because they have an in-depth understanding of oral infectious diseases,” said Fine.
Other Rutgers authors included Theresa Chang and Chuan Xu at the Public Health Research Institute based at Rutgers New Jersey Medical School and Kenneth Markowitz and Carla Cugini at Rutgers School of Dental Medicine.
Two research papers highlight single-cell dissection of kidney tumors to identify new immunotherapy treatments and targets
Newswise — BOSTON - In the last two decades, immunotherapy has emerged as a leading treatment for advanced renal carcinoma cancer (more commonly known as kidney cancer). This therapy is now part of the standard of care, but it doesn’t work for all patients, and almost all patients, no matter how they respond initially, become more resistant to treatment over time. The immune system plays a critical role in kidney cancer disease progression and in response to therapies, and so a fundamental challenge in the field is to understand the underlying “immune circuitry” of this disease.
In two new studies published today in Cancer Cell, researchers from Dana-Farber Cancer Institute and the Broad Institute of MIT and Harvard used the emerging technology of single-cell RNA sequencing to draw a clearer picture of how kidney tumors’ microenvironments change in response to immunotherapy. The researchers believe that this work points to potential targets for new drug therapies.
“We have a standard of care for treating kidney cancer patients, but many patients do not respond to existing therapies, and we need to discover new targets,” said Eliezer Van Allen, MD, an oncologist at Dana-Farber, associate professor of medicine at Harvard Medical School, associate member at the Broad Institute, and co-senior author on one of the papers.
“These companion studies shed important new light on the biology of advanced kidney tumors and their surrounding environments. With this increased understanding, researchers will be able to identify new potential drug treatment targets and, overall, expand the number of patients who can receive effective treatment,” remarked Catherine J. Wu, MD, chief of the Division of Stem Cell Transplantation and Cellular Therapies at Dana-Farber, professor of medicine at Harvard Medical School, an institute member at the Broad, and co-senior author on one of the papers.
“A patient’s immune system plays a critical role in controlling both the progression of cancer and the response to immune therapies,” adds Toni K. Choueiri, MD director of the Lank Center for Genitourinary Oncology at Dana-Farber, an associate member at the Broad, and the Jerome and Nancy Kohlberg Professor of Medicine at Harvard Medical School. Choueiri is co-senior author on both papers. “We don’t quite know why some tumors respond and some don’t. We also don’t know why kidney cancers become resistant to immunotherapy. These two studies are a large team effort to give us a sharper image of what happens on not just the cellular level but down to the RNA of each of those cells.”
With immunotherapy, patients are typically given an immune checkpoint blockade (ICB) (often in combination with VEGF tyrosine kinase inhibitors; TKIs). The drugs are designed to stop the immune system from stopping itself, thus allowing it to attack the tumor like any other unwanted pathogen. However, immunotherapy is only successful in about half of ccRCC patients, and almost all patients build resistance to the treatment over time.
About 76,000 Americans are diagnosed with kidney cancer in the U.S. each year, which is also responsible for more than 13,000 deaths annually, according to the American Cancer Society.
Finding new targets to disrupt an immune dysfunction circuit
In one study, researchers performed single-cell RNA and T cell receptor sequencing on 164,722 individual cells from tumor and adjacent non-tumor tissue. These samples came from 13 patients with clear cell renal cell carcinoma (ccRCC), which make up 80 percent of kidney cancer cases, at different stages of disease: early, locally advanced and advanced/metastatic.
In most solid tumors, the presence of a specific type of immune cell, the CD8+ T cell is a good thing. Their presence shows the immune system is working. However, researchers found that in advanced stage disease these CD8+ T cells were “exhausted,” and not able to carry out their usual function.
They also discovered more anti-inflammatory or “M2-like” macrophages, a type of white blood cell that suppresses the immune system, in advanced stage disease. CD8+ T cells and macrophages were playing off each other and caught in an “immune dysfunction circuit,” said co-lead author David A. Braun, MD, PhD, an oncologist at Dana-Farber and instructor of medicine at Harvard Medical School. In advanced disease samples, macrophages produce molecules that support CD8+ T cell exhaustion, at the same time those CD8+ T cells make molecules that supported the life of pro-tumor macrophages.
These findings are important because they “open up a whole new landscape of potential treatment targets,” said Braun. “We already target some of the immune system pathways in kidney cancer, but our work uncovered many other immune inhibitory pathways supporting cell dysfunction. As we move forward, we can look at all of these interactions and identify new opportunities to disrupt the circuit, with the goal of restoring the immune system’s anti-tumor effect and ultimately improving outcomes for patients with kidney cancer.”
Choueiri and Wu are co-senior authors on the study, “Progressive immune dysfunction with advancing disease stage in renal cell carcinomas.”
Identifying treatments beyond the PD-1/PD-L1 axis
The other study published today looks at tumor and immune reprogramming during immunotherapy in ccRCC.
Most current immunotherapy treatments for ccRCC target the PD-1/PD-L1 axis, a pathway that makes proteins that halt the immune system from attacking cancer cells. Stop the stoppers, and the immune system can go after cancer cells.
But these drugs are only effective in half of ccRCC patients, and almost all patients eventually develop resistance to the drug.
“There may be immune evasion mechanisms outside of PD-1/PD-L1 that play an important role in response or resistance,” said Kevin Bi, computational biologist at Dana-Farber and co-lead author on the paper.
Researchers used single-cell RNA sequencing to look 34,326 total cells drawn from samples from eight patients, seven of whom had metastatic renal cancer and one with localized disease. Five samples were from patients who had already received treatment, either through ICB, or a combination of ICB and TKI. Those treated with ICB were all given drugs that specifically targeted the PD-1/PD-L1 axis.
Researchers found that ICB remodels the cancer microenvironment and changes how cancer and immune cells interact, in a few ways:
In patients whose cancer responded to treatment, subsets of cytotoxic T-cells, which are cancer-fighting lymphocytes, express higher levels of co-inhibitory receptors and effector molecules.
Macrophages from treated biopsies shift towards pro-inflammatory states in response to an interferon-rich microenvironment but also upregulate immunosuppressive markers.
In cancer cells treated with ICB, researchers found two subpopulations, differing in angiogenic signaling and upregulation of immunosuppressive programs.
In advance stage cancers treated with ICB, expression signatures for cancer cell subpopulations and immune evasion were associated with the PBRM1 mutation, the second most commonly mutated gene in ccRCC.
These findings show the importance of exploring immune pathways away from the PD-1/PD-L1 axis, said Meng Xiao He, a graduate student in the Harvard Biophysics program, member of the Van Allen lab at Dana-Farber, and a co-lead author on the paper.
“We need to look at things that are not just CD8+ T cells. We should look at macrophages, some of the other immune checkpoints, and assess what may be targetable,” he said. “We’re still in the early days of trying to understand the mechanisms of immunotherapy resistance in different diseases. There’s a lot of room to keep trying so that more people respond, and those responses hold.”
Choueiri and Van Allen are co-senior authors on the study, "Tumor and immune reprogramming during immunotherapy in advance renal cell carcinoma."
The co-authors of “Progressive immune dysfunction with advancing disease stage in renal cell carcinomas” are Kelly Street, PhD, of Dana-Farber and the Harvard T.H. Chan School of Public Health; Kelly P. Burke, MD, PhD, Dana-Farber and Harvard Medical School; David L. Cookmeyer, of Harvard Medical School; Thomas Denize, MD of Harvard Medical School and Brigham and Women’s Hospital (BWH); Christina B. Pedersen, of Technical University of Denmark, Rigshospitalet-Copenhagen University Hospital; Satyen H. Gohil, PhD, of Dana-Farber, Harvard Medical School, the Broad Institute and University College, London; Nicholas Schindler, BSE, of Dana-Farber; Lucas Pomerance, BA of Dana-Farber and Harvard Medical School; Lauren Hirsch, MD, of Dana-Farber and Harvard Medical School; Ziad Bakouny, MD, of Dana-Farber; Yue Hou, PhD, of Dana-Farber; Juliet Forman, of Dana-Farber and the Broad Institute; Teddy Huang, PhD of Dana-Farber; Shuqiang Li, PhD, of Dana-Farber and Harvard Medical School; Ang Cui, MS, of the Broad Institute and Harvard-MIT Division of Health Sciences and Technology; Derin B. Keskin, PhD, of Dana-Farber and the Broad Institute; John Steinharter, MS, of Dana-Farber; Gabrielle Bouchard, BS, of Dana-Farber; Maxine Sun, PhD, MPH, of Dana-Farber; Erica M. Pimenta, MD, PhD, of Dana-Farber and Harvard Medical School; Wenxin Xu, MD of Dana-Farber and Harvard Medical School; Kathleen M. Mahoney, MD, PhD, of Dana-Farber, Harvard Medical School and Beth Israel Deaconess Medical Center; Bradley A. McGregor, MD, of Dana-Farber and Harvard Medical School; Michelle S. Hirsch, MD, PhD, of Harvard Medical School and BWH; Steven L. Chang, MD, of Harvard Medical School and BWH; Kenneth J. Livak, PhD, of Dana-Farber; David F. McDermott, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center; Sachet A. Shukla, PhD, of the Broad Institute and Dana-Farber; Lars R. Olsen, PhD of Technical University of Denmark, Center for Genomic Medicine, Rigshospitalet; Sabina Signoretti, MD, of Harvard Medical School, BWH and Dana-Farber; Arlene H. Sharpe, MD, PhD, of the Broad Institute, Harvard Medical School and BWH; Rafael A. Irizarry, PhD, of Dana-Farber and Harvard T.H. Chan School of Public Health.
Financial support was provided Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Program of Research Excellence (grants P50CA101942-12 and P50CA101942); Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Program of Research Excellence Career Enhancement Program (grants P50CA101942-15 and P50CA101942), Department of Defense Congressionally Directed Medical Research Programs (grants KC170216 and KC190130); Department of Defense Academy of Kidney Cancer Investigators (grant KC190128), National Institute of General Medical Sciences (grants 5R35GM131802 and 5R01GM083084), National Human Genome Research Institute ENCODE Data Analysis Center (grant 5R01HG009446), Independent Research Fund Denmark (grant 8048-00078B), Kay Kendall Leukemia Fund Fellowship, Foundation de France during her post-doctoral, Bristol-Myers Squibb, Genentech, National Cancer Institute Research Specialist Award (grant R50CA251956), National Cancer Institute (grants R21 CA216772-01A1 and NCI-SPORE-2P50CA101942-11A1), ASCO Conquer Cancer Foundation Young Investigator Award; National Cancer Institute (grant R50RCA211482); National Human Genome Research Institute (grant R35GM131802), Cancer Center Support Grant (P30CA006516), Kohlberg Chair at Harvard Medical School, Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber; National Institute of Health (grants NCI-1RO1CA155010 and NIH/NCI U24 CA224331); G. Harold and Leila Y. Mathers Foundation and the Parker Institute for Cancer Immunotherapy.
The co-authors of “Tumor and immune reprogramming during immunotherapy in advance renal cell carcinoma” are Ziad Bakouny, MD, of Dana Farber; Abhay Kanodia, Sara Napolitano, Jingyi Wu, and Grace Grimaldi, BS, of Dana-Farber and the Broad Institute; David A. Braun, MD, PhD, of Dana-Farber, the Broad Institute and Harvard Medical School; Michael S. Cuoco, BS, of the Broad Institute; Angie Mayorga, BA, of Dana-Farber; Laura DelloStritto, MPH, of Dana-Farber and the Broad Institute; Gabrielle Bouchard, BS, of Dana-Farber; John Steinharter, MS, of Dana-Farber; Alok K. Tewari, MD, PhD, of Dana-Farber and Harvard Medical School; Natalie I. Vokes, MD, of Dana-Farber and the Broad Institute; Erin Shannon, BS, of the Broad Institute; Maxine Sun, PhD, MPH of Dana-Farber; Jihye Park, PhD, of Dana-Farber and the Broad Institute; Steven L. Chang, MD, of BWH; Bradley A. McGregor, MD, of Dana-Farber; Rizwan Haq, MD, PhD of Dana-Farber and the Broad Institute; Thomas Denize, MD, of Harvard Medical School and BWH; Sabina Signoretti, MD, of Harvard Medical School, BWH and Dana-Farber; Jennifer L. Guerriero, PhD, of Harvard Medical School and Dana-Farber; Sébastien Vigneau, PhD, of Dana-Farber and the Broad Institute; Orit Rozenblatt-Rosen, PhD, of the Broad Institute; Asaf Rotem, PhD, of Dana-Farber and the Broad Institute; Aviv Regev, PhD, of Genentech.
Financial support was provided by the National Institutes of Health (grants U01 CA233100, R01 CA227388, U2C CA233195, T32 GM008313, T32 CA009172); the National Science Foundation (grant GRFP DGE1144152), Novartis-DDP grant, Kure It-AACR grant, Dunkin’ Donuts Breakthrough Grant, Dana-Farber/Harvard Cancer Center Kidney Cancer Specialized Program of Research Excellence (grant P50CA101942-15; Department of Defense Congressionally Directed Medical Research Programs (grants KC170216 and KC190130), Department of Defense Academy of Kidney Cancer Investigators (grant KC190128), Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber.
About Dana-Farber Cancer Institute
Dana-Farber Cancer Institute is one of the world’s leading centers of cancer research and treatment. Dana-Farber’s mission is to reduce the burden of cancer through scientific inquiry, clinical care, education, community engagement, and advocacy. We provide the latest treatments in cancer for adults through Dana-Farber/Brigham and Women’s Cancer Center and for children through Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. Dana-Farber is the only hospital nationwide with a top 10 U.S. News & World Report Best Cancer Hospital ranking in both adult and pediatric care.
As a global leader in oncology, Dana-Farber is dedicated to a unique and equal balance between cancer research and care, translating the results of discovery into new treatments for patients locally and around the world, offering more than 1,100 clinical trials.