News
Newswise — COLUMBUS, Ohio – A simple addition to injected COVID-19 vaccines could enhance their effectiveness and provide “border protection” immunity in areas like the nose and mouth to supplement antibodies in the bloodstream, new research suggests.
The strategy involves dampening the activity of an enzyme produced by some white blood cells when they’re responding to the vaccine challenge. When highly active, this enzyme breaks down not just the pathogen – its job – but also degrades pieces of cells that participate in the immune response.
Research in mice showed that an experimental COVID-19 vaccine containing a compound to inhibit the enzyme stimulated a robust antibody response that included immunity in the nose and mouth, ultimately providing extra protection for airways and the gastrointestinal tract.
“Our approach is to improve ‘border control.’ The benefits are broad because in addition to providing protection in the bloodstream like most vaccines do, we also have excellent protection in the doors and windows of the body that communicate with the outside,” said senior study author Prosper Boyaka, professor and chair of the Department of Veterinary Biosciences at The Ohio State University.
“If we protect the mucosal area where the pathogen enters, then even if you don’t reach total immunity there, you limit the amount of pathogen that enters the body so the antibodies inside are more efficient at clearing the infection.”
The experimental vaccine was produced by packaging a segment of the SARS-CoV-2 (the virus that causes COVID-19) spike protein as an antigen with the common vaccine ingredient aluminum salts and an enzyme inhibitor. The findings suggest this affordable design could be particularly helpful in developing countries, where cold storage needed for existing vaccines is a challenge, said Boyaka, also an investigator and program director in Ohio State’s Infectious Diseases Institute.
The study was published online back on Aug. 5 in Proceedings of the National Academy of Sciences.
There is an irony to the use of aluminum salts (also known as alum) in about 70% of the world’s vaccines: While alum’s presence actually enhances the immune response, it also recruits the white blood cells that secrete the enzyme, called elastase.
Alum is inexpensive to obtain or produce and can be stored at room temperature, and is effective at promoting development of a bloodstream-based antibody response to vaccination. But it doesn’t do much for cell-mediated immunity that improves protection against viruses and bacteria that use cells to reproduce, and can’t generate a useful number of antibodies in the body’s portals of entry for most pathogens: the nose, mouth and genitourinary tract.
The researchers found that suppressing elastase in a vaccine containing alum had the dual benefits of broadening and speeding up the antibody response in the bloodstream and triggering the specific types of antibodies needed for immune protection of mucous membranes.
“We found a way to have the cells come and help the immune response to develop and the enzyme to break down the pathogen, but we don’t want that response to be so high that it goes out of control. So we’re just putting a brake on the activity those enzymes would have,” Boyaka said. “And we found if you apply that strategy, you can induce a response in the airways even if the vaccine is not given through the airway.”
The experimental vaccine enhanced the magnitude of mouse antibodies, which reacted to the same section of the spike protein in the vaccine that antibodies in plasma from COVID-19 patients attach to, as well as generating antibodies in mucosal areas. Immunized mice lacking the gene for the enzyme developed high-affinity antibodies as well.
To further test the concept, the researchers found the enzyme-suppressing compound used in the study triggered production of specialized inflammation-regulating cells in cultures of human immune cells and pig spleen cells, showing that this strategy could improve vaccine immune responses in other species – including people.
Boyaka’s team envisions that a future injected vaccine containing an elastase inhibitor could expand SARS-CoV-2 vaccination availability across the world and even be used to boost existing vaccines.
“COVID will stay with us for some time, unfortunately, with the new variants,” he said. “What we need to do is have a portfolio of options that we could use depending on the health environment.
“Reprogramming the immune response induced by an injected vaccine containing alum is a way to make the vaccine more efficient for what we need. This could be a cheap and simple approach that can benefit people in developing countries.”
This work was supported by grants from the National Institutes of Health and an Ohio State Office of Research COVID-19 seed grant. A patent application has been filed spanning this research; the overall patent portfolio includes an additional U.S. issued patent.
Co-authors, all from Ohio State, include Eunsoo Kim, Zayed Attia, Rachel Woodfint, Cong Zeng, Sun Hee Kim, Haley Steiner, Rajni Kant Shukla, Namal Liyanage, Shristi Ghimire, Jianrong Li, Gourapura Renukaradhya, Abhay Satoskar, Amal Amer, Shan-Lu Liu and Estelle Cormet-Boyaka.
Newswise — GRAND RAPIDS, Mich. (August 3, 2021) — The average American eats roughly 22 teaspoons of added sugar a day — more than three times the recommended amount for women and more than double the recommended amount for men.
Although this overconsumption is known to contribute to Type 2 diabetes and other disorders, the exact ways in which eating too much sugar sets the stage for metabolic diseases on a cellular level has been less clear.
Now, a team led by Van Andel Institute scientists has found that surplus sugar may cause our cellular powerplants — called mitochondria— to become less efficient, reducing their energy output.
The findings, published today in Cell Reports, highlight the cellular implications of excessive sugar consumption and provide an important new model to study the initial metabolic events that may contribute to diabetes development.
“The body needs sugar, or glucose, to survive, but, as the saying goes: ‘All good things in moderation,’” said Ning Wu, Ph.D., an assistant professor at Van Andel Institute and corresponding author of the study. “We found that too much glucose in cells, which is directly linked to the amount of sugar consumed in one’s diet, affects lipid composition throughout the body, which in turn affects the integrity of mitochondria. The overall effect is a loss of optimal function.”
Using their new model, Wu and her colleagues demonstrated that excess glucose reduces the concentration of polyunsaturated fatty acids (PUFAs) in the mitochondrial membrane and makes mitochondria less efficient. PUFAs are vital players in supporting mitochondrial function and mediating a host of other biological processes such as inflammation, blood pressure and cellular communication.
Instead, excess glucose is synthesized into a different form of fatty acid that isn’t as efficient or as flexible as PUFAs. This upends the lipid composition of the membrane and puts stress on the mitochondria, damaging them and impacting their performance.
Wu and her colleagues were able to reverse this detrimental effect by feeding their mouse models a low-sugar ketogenic diet, which suggests that reducing glucose and restoring normal membrane lipid composition supports healthy mitochondrial integrity and function. They also found that consuming excess carbohydrates reduces the beneficial effect of PUFA supplements.
“Although we may not always notice the difference in mitochondrial performance right away, our bodies do,” Wu explained. “If the lipid balance is thrown off for long enough, we may begin to feel subtle changes, such as tiring more quickly. While our study does not offer medical recommendations, it does illuminate the early stages of metabolic disease and provides insights that may shape future prevention and therapeutic efforts.”
Other authors include Althea N. Waldhart, Brejnev Muhire, Ph.D., Ben Johnson, Ph.D., Dean Pettinga, Zachary B. Madaj, M.S., Emily Wolfrum, MPH, Vanessa Wegert, and J. Andrew Pospisilik, Ph.D., of VAI; and Xianlin Han, Ph.D., of the Sam and Ann Barshop Institute for Longevity and Aging Studies and the Department of Medicine at UT Health San Antonio.
Research reported in this publication was supported by Van Andel Institute; the National Institute of General Medical Sciences of the National Institutes of Health under award no. R01GM120129 (Wu); and the National Institute on Aging of the National Institutes of Health under award no. RF1AH061872 (Han). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
A 27-question survey of Association of American Cancer Institutes (AACI) members, including many NCI-designated centers, identified several opportunities to improve coordination of care between main centers and their satellite locations.
Newswise — PLYMOUTH MEETING, PA [July 6, 2021] — New research in the June 2021 issue of JNCCN—Journal of the National Comprehensive Cancer Network assesses the quality of cancer care delivered through extended sites coordinated by some of the country’s largest cancer centers. The study was developed to implement strategies for disseminating discoveries and expanding access to the highest quality cancer care as part of AACI’s Network Care Initiative, established by former AACI President Stanton L. Gerson, MD, Director of the Case Comprehensive Cancer Center. Results were calculated based on responses to a mixed-methods survey answered by 69 cancer centers between September 2017 and December 2018, at which time 56 reported at least one network practice site.
Just over half indicated that network sites had full access to the main centers’ electronic medical records (EMRs), and even fewer main centers had complete access to records throughout their network sites.
“Our findings demonstrate the need to improve network site alignment, particularly in patient navigators, care paths, and clinical trial access,” said Dr. Gerson, the study’s lead researcher and interim dean of the Case Western Reserve University School of Medicine. “Most federal cancer center reviews do not assess the total population of cancer patients served by major cancer centers and their affiliated sites. These data suggest that a very sizable portion of new cancer cases are cared for by these centers and their networks. Greater cancer center/network coordination could ultimately lead to improved access to clinical trials for the underrepresented communities many of these network sites serve.”
According to the survey results, some key opportunities to improve coordination of care include:
Implementing integrated EMRs across networks;
Reviewing best clinical care practices, with more rigorous use of care paths and coordination of diagnosis and treatment planning across sites;
Greater attention and support for cancer clinical trials across network sites; and
Improved physician oversight of clinical and research expectations, hiring, review and other links with cancer center main campus sites.
“Many studies show that consistency through care plans and guidelines improves patient outcomes, clinical response, and survival. More proactive approaches, including care paths, tumor boards across networks, and recognition of the value of placing disease experts at network sites, will improve the standardization of care across sites,” Dr. Gerson added.
“Disparities in cancer care outcomes, most significantly patient survival, have been shown between NCI-designated cancer centers and community hospitals, where two-thirds of cancer patients are cared for in the U.S.” commented Lawrence N. Shulman, MD, Deputy Director for Clinical Services at the Abramson Cancer Center at the University of Pennsylvania, who was not involved in this research. “Rural cancer programs often have limited cancer physicians representing all relevant specialties and urban safety-net hospitals often have limited financial resources to support high-quality cancer programs. Partnerships between academic cancer centers and community and safety-net hospitals have the potential to improve outcomes for a broader spectrum of cancer patients in the U.S. One might consider support of these cancer programs an obligation of academic cancer centers. This study outlines some potential mechanisms of support.”
The COVID-19 pandemic, which began well after this survey closed, and the growing call for increasing diversity in clinical trials, is also driving the need to better integrate network sites as a tool for delivering quality care to underserved populations. On June 7, NCCN presented a webinar on “Utilization of Network Satellite Locations” as part of a series on COVID-19 and Cancer Center Operations. Dr. Shulman was one of the panelists, along with other members of the NCCN Best Practices Committee. That video is available at: NCCN.org/covid-19.
To read the entire study, visit JNCCN.org. Complimentary access to “Status of Cancer Care at Network Sites of the Nation’s Academic Cancer Centers” is available until September 10, 2021.
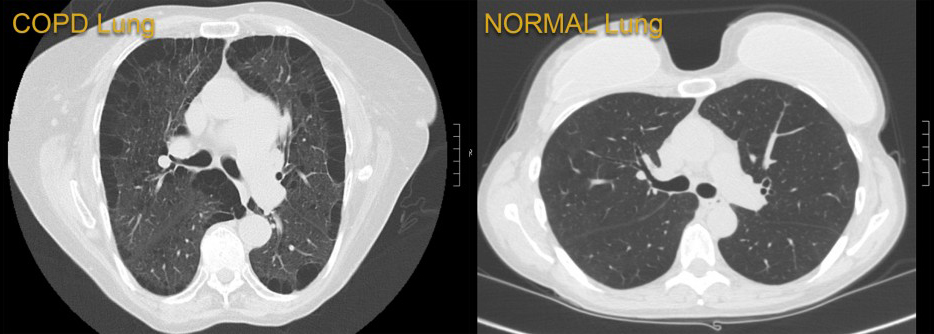
Newswise — BALTIMORE (June 14, 2021) – Researchers at the University of Maryland School of Medicine (UMSOM) analyzed data at the 13-hospital University of Maryland Medical System (UMMS) and found public health measures designed to reduce the spread of the COVID-19 virus may have fostered a substantial side benefit: Hospital admissions for chronic obstructive pulmonary disease (COPD) were reduced by 53 percent, according to a new study published in The American Journal of Medicine. This is likely due to a drop in circulating seasonal respiratory viruses such as influenza.
Hospitalizations for COPD, a group of lung diseases that make it hard to breathe and get worse over time, are commonly driven by flare-ups where symptoms are triggered by such factors as tobacco smoke, air pollution and respiratory infections. Seasonal respiratory viruses, including those that cause the common cold or influenza, trigger nearly half of those flare-ups.
In the wake of a marked drop in COPD admissions during the pandemic, the researchers theorized that COVID-19 behavior changes – a mix of stay-at-home orders, social distancing, masking mandates and strict limitations on large gatherings – not only protected against COVID-19, but they may have also reduced exposure to other respiratory infections.
Conversely, they worry that the return to normal behavior may lead to more COPD flare-ups.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert M. Reed, MD, UMSOM Professor of Medicine and pulmonologist at the University of Maryland Medical Center (UMMC). “If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back. There could be a lot of illness.”
Prior to the COVID-19 pandemic, COPD was the fourth-leading cause of death worldwide and a leading cause of hospital admissions in the United States. The pandemic has led to significant changes in health care delivery, including reduced admissions for COPD and other non-COVID illnesses, some of which may have stemmed from patients’ fear of contracting COVID in various hospital settings, as well as a shift toward telemedicine and outpatient COPD management during the pandemic.
To understand what may have occurred to reduce COPD admissions, the researchers compared weekly hospital admissions for COPD in the pre-COVID-19 years of 2018 and 2019, with admissions after the COVID-19 public health measures were instituted. At UMMS, those measures were implemented before April 1, 2020, so the investigators chose the same five-month period in each year for their comparison, April 1 to Sept. 30.
Co-lead author Jennifer Y. So, MD, UMSOM Assistant Professor of Medicine and COPD specialist at UMMC, said electronic medical records from multiple hospitals across a range of communities in the UMMS database facilitated a granular evaluation of changes over time. “We assessed a variety of possible causes that could affect COPD admissions including the presence of multiple diseases or medical conditions and the frequency of COPD exacerbations.”
The database findings were correlated with data on respiratory viral trends from the U.S. Centers for Disease Control and Prevention for the period of Jan. 1, 2018, through Oct. 1, 2020.
“We found a 53 percent drop in COPD admissions throughout UMMS during COVID-19. That is substantial, but equally significant, the drop in weekly COPD admissions was 36 percent lower than the declines seen in other serious medical conditions, including congestive heart failure, diabetes and heart attack,” said Dr. So.
As more and more people are vaccinated against COVID-19 and many of the public health measures of the past year are relaxed, the researchers warn that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“Our study did not assess which public health components worked to tame seasonal respiratory viruses, but a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure,” said Dr. Reed.
Dr. So, who is from South Korea, said it is a cultural norm to wear masks during the winter in her native country. “The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said.
“This is a compelling study that raises some important public health questions about protecting our most vulnerable patient populations after we are finished with the COVID-19 pandemic. I certainly think it warrants a fuller discussion,” said UMSOM Dean E. Albert Reece, MD, PhD, MBA, University Executive Vice President for Medical Affairs and the John Z. and Akiko K. Bowers Distinguished Professor.
So JY, O’Hara NN, Kenaa B, Williams JG, deBorja CL, Slejko JF, Zafari Z, Sokolow M, Zimand P, Deming M, Marx J, Pollak A, Reed RM. Decline in COPD Admissions During the COVID-19 Pandemic Associated with Lower Burden of Community Respiratory Viral Infections. The American Journal of Medicine, June 11, 2021. doi: https://doi.org/10.1016/j.amjmed.2021.05.008
Newswise — Washington, DC (June 1, 2021) — Many individuals with kidney failure have been unable to self-isolate during the COVID-19 pandemic because they require dialysis treatments in clinics several times a week. New research that will appear in an upcoming issue of CJASN highlights the risks faced by these patients and the factors involved.
For the study, Ben Caplin, MBChB, PhD (University College London) and his colleagues, on behalf of the Pan-London COVID-19 Renal Audit Group, examined information on 5,755 patients who received dialysis in 51 clinics in London. Between March 2 and May 31, 2020, a total of 990 (17%) patients tested positive and 465 (8%) were admitted to hospitals with suspected COVID-19. COVID-19 risks were higher in patients who were older, had diabetes, lived in local communities with higher COVID-19 rates, and received dialysis at dialysis clinics that served a larger number of patients. Risks were lower in patients who received dialysis in clinics with a higher number of available side rooms and that had mask policies for asymptomatic patients. No independent association was seen with sex, ethnicity, or measures of deprivation.
“Taken together, the findings confirm the high rates of symptomatic COVID-19 among patients receiving in-center dialysis and suggest sources of transmission both within dialysis units and patients’ home communities,” said Dr. Caplin. “The work also suggests that in addition to isolation of confirmed cases, addressing factors that might reduce transmission from patients without suspected or confirmed disease might provide an additional opportunity to further modify the impact of COVID-19 in this population.”
Study co-authors include Damien Ashby, Kieran McCafferty, Richard Hull, Elham Asgari, Martin L. Ford, Nicholas Cole, Marilina Antonelou, Sarah A. Blakey, Vinay Srinivasa, Dandisonba C.B. Braide-Azikwe, Tayeba Roper, Grace Clark, Helen Cronin, Nathan J. Hayes, Bethia Manson, Alexander Sarnowski, Richard Corbett, Kate Bramham, Eirini Lioudak7, Nicola Kumar, Andrew Frankel, David Makanjuola, Claire C. Sharpe, Debasish Banerjee, and Alan D. Salama.
Disclosures: Dr. Caplin reports personal fees from Lifearc, grants from Astra Zeneca, grants from Colt Foundation, grants from Medical Research Council, from Royal Free Charity, outside the submitted work; Dr. Ashby reports personal fees from Fibrogen, outside the submitted work; Dr. Corbett has a patent WO2017148836A1: "A device for maintaining vascular connections" issued; Dr. Banerjee reports grants from AstraZeneca, grants from Kidney Research UK, personal fees from ViforPharma, outside the submitted work.
The article, titled “Risk of COVID-19 Disease, Dialysis Unit Attributes, and Infection Control Strategy among London In-Center Hemodialysis Patients,” will appear online at http://cjasn.asnjournals.org/ on June 1, 2021.
The content of this article does not reflect the views or opinions of The American Society of Nephrology (ASN). Responsibility for the information and views expressed therein lies entirely with the author(s). ASN does not offer medical advice. All content in ASN publications is for informational purposes only, and is not intended to cover all possible uses, directions, precautions, drug interactions, or adverse effects. This content should not be used during a medical emergency or for the diagnosis or treatment of any medical condition. Please consult your doctor or other qualified health care provider if you have any questions about a medical condition, or before taking any drug, changing your diet or commencing or discontinuing any course of treatment. Do not ignore or delay obtaining professional medical advice because of information accessed through ASN. Call 911 or your doctor for all medical emergencies.
Since 1966, ASN has been leading the fight to prevent, treat, and cure kidney diseases throughout the world by educating health professionals and scientists, advancing research and innovation, communicating new knowledge, and advocating for the highest quality care for patients. ASN has more than 21,000 members representing 131 countries. For more information, visit www.asn-online.org.
Newswise — Within the next decade, the novel coronavirus responsible for COVID-19 could become little more than a nuisance, causing no more than common cold-like coughs and sniffles. That possible future is predicted by mathematical models that incorporate lessons learned from the current pandemic on how our body’s immunity changes over time. Scientists at the University of Utah carried out the research, now published in the journal Viruses.
“This shows a possible future that has not yet been fully addressed,” says Fred Adler, PhD, professor of mathematics and biological sciences at the U. “Over the next decade, the severity of COVID-19 may decrease as populations collectively develop immunity.”
The findings suggest that changes in the disease could be driven by adaptations of our immune response rather than by changes in the virus itself. Adler was senior author on the publication with Alexander Beams, first author and graduate student in the Department of Mathematics and the Division of Epidemiology at University of Utah Health, and undergraduate co-author Rebecca Bateman.
Although SARS-CoV-2 (the sometimes-deadly coronavirus causing COVID-19) is the best-known member of that virus family, other seasonal coronaviruses circulate in the human population—and they are much more benign. Some evidence indicates that one of these cold-causing relatives might have once been severe, giving rise to the “Russian flu” pandemic in the late 19th century. The parallels led the U of U scientists to wonder whether the severity of SARS-CoV-2 could similarly lessen over time.
To test the idea, they built mathematical models incorporating evidence on the body’s immune response to SARS-CoV-2 based on the following data from the current pandemic.
There is likely a dose response between virus exposure and disease severity.
A person exposed to a small dose of virus will be more likely to get a mild case of COVID-19 and shed small amounts of virus.
By contrast, adults exposed to a large dose of virus are more likely to have severe disease and shed more virus.
Masking and social distancing decrease the viral dose.
Children are unlikely to develop severe disease.
Adults who have had COVID-19 or have been vaccinated are protected against severe disease.
Running several versions of these scenarios showed that the three mechanisms in combination set up a situation where an increasing proportion of the population will become predisposed for mild disease over the long term. The scientists felt the transformation was significant enough that it needed a new term. In this scenario, SARS-CoV-2 would become “Just Another Seasonal Coronavirus,” or JASC for short.
“In the beginning of the pandemic, no one had seen the virus before,” Adler explains. “Our immune system was not prepared.” The models show that as more adults become partially immune, whether through prior infection or vaccination, severe infections all but disappear over the next decade. Eventually, the only people who will be exposed to the virus for the first time will be children—and they’re naturally less prone to severe disease.
“The novel approach here is to recognize the competition taking place between mild and severe COVID-19 infections and ask which type will get to persist in the long run,” Beams says. “We’ve shown that mild infections will win, as long as they train our immune systems to fight against severe infections.”
The models do not account for every potential influence on disease trajectory. For example, if new virus variants overcome partial immunity, COVID-19 could take a turn for the worse. In addition, the predictions rely on the key assumptions of the model holding up.
“Our next step is comparing our model predictions with the most current disease data to assess which way the pandemic is going as it is happening,” Adler says. “Do things look like they’re heading in a bad or good direction? Is the proportion of mild cases increasing? Knowing that might affect decisions we make as a society.”
Newswise — Before undergoing surgery, patients often go through a number of tests: blood work, sometimes a chest X-ray, perhaps tests to measure heart and lung function.
In fact, about half of patients who had one of three common surgical procedures done in Michigan between 2015 and the midway point of 2019 received at least one routine test beforehand.
That’s according to new research in JAMA Internal Medicine from a collaboration between the University of Michigan-based Michigan Program on Value Enhancement (MPrOVE) and the Michigan Value Collaborative, a statewide initiative that focuses on improving medical and surgical quality.
Yet plenty of evidence suggests that preoperative testing is often unnecessary for low-risk surgeries.
At best, it’s costly and doesn’t usually improve outcomes for patients. At worst, it can lead to more invasive testing and delay surgery, which can create complications that could have been avoided if the tests weren’t done.
“There aren’t that many areas in medicine where the data is pretty definitive that something is low-value,” says Lesly Dossett, M.D., the division chief of surgical oncology at Michigan Medicine and the co-director of MPrOVE, “but preoperative testing before low-risk surgeries is certainly one of them.”
How testing started — and why it continues
In the latter half of the 19th century, modern surgery was still in its infancy.
Anesthesia was new, and even minor surgeries were not routine. So researchers used tests to assess their patients’ physical health and measure their risk of complications during operations.
“There was probably a time when some of the testing did reduce adverse events,” Dossett says. “But now there’s been so many advances in surgery — complication rates are so low that a lot of these tests are not necessarily helpful anymore.”
Others agree with her.
Professional organizations ranging from the American College of Surgeons and the Society of General Internal Medicine to the American Society of Anesthesiologists have identified routine preoperative testing as a low-value type of care that should be reduced whenever possible.
In 2012, the American Board of Internal Medicine Foundation even launched an initiative called the Choosing Wisely campaign that promotes conversations between health care providers and patients about unnecessary medical tests and procedures.
But, almost a decade later, preoperative tests continue to be ordered.
Of about 40,000 patients in the U-M study who had surgery to either remove the gall bladder, repair a groin hernia, or remove cancerous breast tissue, close to a third underwent two or more tests beforehand, and about 13% had three or more. The most common tests were a complete blood count, an electrocardiogram and a basic metabolic panel, all of which aren’t inherently necessary before these surgeries.
“It’s one thing to say that this is well recognized in the literature,” says Hari Nathan, M.D., Ph.D., who happens to be the division chief of hepato-pancreato-biliary surgery at Michigan Medicine as well as the director of the MVC, “but it’s a different thing to put it in the hands of the clinicians who are at the bedside in an easy-to-read, easy-to-understand and convenient-to-carry-around format.”
Patients who had a complete medical history and physical done during a visit that was separately billed were more likely to have had preoperative testing as were those who were older or had more than one medical condition.
“I could see those two latter factors being in the background, hypothetically giving some pressure to do more testing,” says Nicholas Berlin, M.D., M.P.H., a plastic surgery resident at Michigan Medicine and the first author of the study. “That’s not to suggest there’s an age threshold or a comorbidity that requires preoperative testing every single time. There’s not.”
A small number of people who fall into these categories may actually benefit from having these tests done, although it’s difficult to know exactly how many based on this data, the researchers say. Yet, when they adjusted their model to account for that issue, they still found overuse of testing.
The data also revealed wide variations in testing, not only between the 63 hospitals studied but also within health systems for the same procedures, pointing to the need for more research to drill down further into the origins of the problem.
“We have more work to do on our end to figure out what’s driving these differences within and between hospitals,” says Berlin, who’s also a National Clinician Scholar at the University of Michigan Institute for Healthcare Policy and Innovation. “This is signaling to other projects in the future between MPrOVE and statewide quality collaboratives that use more of an on-the-ground approach.”
Value added statewide
This study represents one of the first partnerships between the Michigan Value Collaborative and MPrOVE, a joint venture of IHPI and Michigan Medicine that tries to optimize patient care, improve quality and demonstrate the value of care at Michigan Medicine through research and analytics.
In the past, MPrOVE has worked to reduce preoperative visits and tests such as EKGs before cataract surgery at Michigan Medicine, but its leaders wanted to expand the scope of their research to include other procedures and more hospitals. The MVC was an ideal partner to do so: Funded by Blue Cross Blue Shield of Michigan, the initiative allows more than 90 hospitals and 40 physician organizations in Michigan to compare their data and identify best practices as well as opportunities for improvement.
“It’s something that’s squarely in MVC’s strike zone and fits very well with MPrOVE’s mission,” Nathan says, “It just made sense for us to work together on this.”
Limiting preoperative testing is one of two signature projects for the MVC, and Nathan has already started meeting with area health systems to tackle the issue.
“Some hospitals routinely send patients through a preoperative clinic, which represents a way to move the needle here in a very targeted way, just by influencing what gets ordered in the setting of that clinic,” he says. “At other hospitals, there is no such clinic, and the individual surgeons and/or anesthesiologists are ordering tests, so that might require a different approach in order to get more adherence to guidelines.”
“But I love seeing variation because when we see variation, that means there’s an opportunity to learn from one another,” he adds.
One of the challenges in reducing preoperative testing is that it generates revenue for hospitals, which means there’s not a financial incentive to do less of it, Dossett says. But Nathan says that, based on his interactions with local health systems, he believes there’s an appetite for change in this area.
“At the end of the day, we all recognize that as a society, we need to find ways to curb health care costs,” he says. “That’s in everybody’s interest. Even if, on your balance sheet, you think it makes sense to do more tests just to make money, as health care providers and as a nation, it does not make sense. It is unsustainable. When we talk to our members, everybody gets that.”
Newswise — LEXINGTON, Ky. (May 5, 2021) - Collaborative research between the University of Kentucky (UK) and University of Southern California (USC) suggests that a noninvasive neuroimaging technique may index early-stage blood-brain barrier (BBB) dysfunction associated with small vessel disease (SVD). Cerebral SVD is the most common cause of vascular cognitive impairment, with a significant proportion of cases going on to develop dementia. BBB dysfunction represents a promising early marker of SVD because the BBB regulates a number of important metabolic functions, including clearance of toxic brain substances.
Advanced BBB dysfunction can be detected with neuroimaging measures such as positron emission tomography (PET) scanning and dynamic contrast-enhanced (DCE) MRI. However, these methods require exposure to radiation or contrast agents and may only detect moderate to advanced stages of BBB tissue disruption. The UK-USC study used a novel, noninvasive MRI method called diffusion-prepared arterial spin labeling (DP-ASL), which was developed by Xingfeng Shao, Ph.D. and Danny Wang, Ph.D. at USC. The DP-ASL method indexes subtle BBB dysfunctions associated with altered water exchange rate across the BBB.
In the UK-USC study, healthy older adults (67-86 years old) without cognitive impairment were scanned with the DP-ASL sequence at the UK’s Magnetic Resonance Imaging and Spectroscopy Center. In addition, study participants volunteered for lumbar cerebrospinal fluid (CSF) draw as part of their enrollment in the study at UK’s Sanders-Brown Center on Aging (SBCoA). The study focused on CSF levels of amyloid-beta (Aβ), which are abnormally low when this protein is not adequately cleared from the brain into the CSF.
Results indicated that low CSF levels of Aβ were associated with a low BBB water exchange rate assessed with the DP-ASL method. “Our results suggest that DP-ASL may provide a noninvasive index of BBB clearance dysfunction prior to any detectable cognitive impairment,” said Brian Gold, Ph.D., professor in the UK department of Neuroscience and SBCoA.
Gold is the lead author of the article, which appears in a recent issue of Alzheimer's & Dementia: The Journal of the Alzheimer's Association. Wang, a professor of Neurology and Radiology at USC, the study’s senior author, said, “Our data indicate the important role of BBB water exchange in the clearance of amyloid-beta, and the potential for using DP-ASL to noninvasively assess BBB water exchange in clinical trials of SVD.”
In addition to Gold, several others from UK contributed to the research including Dr. Gregory Jicha, professor in the department of Neurology and SBCoA, Donna Wilcock, Ph.D., professor in the department of Physiology and SBCoA, Tiffany Sudduth and Elayna Seago.
Results from the UK-USC study also support growing evidence that BBB dysfunction may represent a link between SVD and clinical diagnosis of Alzheimer’s disease (AD). Excess accumulation of Aβ is a hallmark feature of individuals who receive a clinical diagnosis of AD. However, Aβ pathology is also seen in many cases of SVD. Results from the UK-USC study are consistent with theories suggesting that insufficient clearance of Aβ through the BBB may impair BBB function which, in turn, may further accelerate the accumulation of Aβ in the brain. Gold noted that “an important topic for future research is why some individuals with BBB dysfunction and impaired Aβ clearance may develop cognitive declines associated with AD while others develop more vascular-like cognitive declines.”
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG055449, National Institute of General Medical Sciences of the National Institutes of Health under Award Number S10OD023573, National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers UH3-NS100614 and R01NS114382, National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB028297.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Newswise — As the COVID-19 pandemic has progressed, it has become clear that many survivors — even those who had mild cases — continue to manage a variety of health problems long after the initial infection should have resolved. In what is believed to be the largest comprehensive study of long COVID-19 to date, researchers at Washington University School of Medicine in St. Louis showed that COVID-19 survivors — including those not sick enough to be hospitalized — have an increased risk of death in the six months following diagnosis with the virus.
The researchers also have catalogued the numerous diseases associated with COVID-19, providing a big-picture overview of the long-term complications of COVID-19 and revealing the massive burden this disease is likely to place on the world’s population in the coming years.
The study, involving more than 87,000 COVID-19 patients and nearly 5 million control patients in a federal database, appears online April 22 in the journal Nature.
“Our study demonstrates that up to six months after diagnosis, the risk of death following even a mild case of COVID-19 is not trivial and increases with disease severity,” said senior author Ziyad Al-Aly, MD, an assistant professor of medicine. “It is not an exaggeration to say that long COVID-19 — the long-term health consequences of COVID-19 — is America’s next big health crisis. Given that more than 30 million Americans have been infected with this virus, and given that the burden of long COVID-19 is substantial, the lingering effects of this disease will reverberate for many years and even decades. Physicians must be vigilant in evaluating people who have had COVID-19. These patients will need integrated, multidisciplinary care.”
In the new study, the researchers were able to calculate the potential scale of the problems first glimpsed from anecdotal accounts and smaller studies that hinted at the wide-ranging side effects of surviving COVID-19, from breathing problems and irregular heart rhythms to mental health issues and hair loss.
“This study differs from others that have looked at long COVID-19 because, rather than focusing on just the neurologic or cardiovascular complications, for example, we took a broad view and used the vast databases of the Veterans Health Administration (VHA) to comprehensively catalog all diseases that may be attributable to COVID-19,” said Al-Aly, also director of the Clinical Epidemiology Center and chief of the Research and Education Service at the Veterans Affairs St. Louis Health Care System.
The investigators showed that, after surviving the initial infection (beyond the first 30 days of illness), COVID-19 survivors had an almost 60% increased risk of death over the following six months compared with the general population. At the six-month mark, excess deaths among all COVID-19 survivors were estimated at eight people per 1,000 patients. Among patients who were ill enough to be hospitalized with COVID-19 and who survived beyond the first 30 days of illness, there were 29 excess deaths per 1,000 patients over the following six months.
“These later deaths due to long-term complications of the infection are not necessarily recorded as deaths due to COVID-19,” Al-Aly said. “As far as total pandemic death toll, these numbers suggest that the deaths we’re counting due to the immediate viral infection are only the tip of the iceberg.”
The researchers analyzed data from the national health-care databases of the U.S. Department of Veterans Affairs. The dataset included 73,435 VHA patients with confirmed COVID-19 but who were not hospitalized and, for comparison, almost 5 million VHA patients who did not have a COVID-19 diagnosis and were not hospitalized during this time frame. The veterans in the study were primarily men (almost 88%), but the large sample size meant that the study still included 8,880 women with confirmed cases.
To help understand the long-term effects of more severe COVID-19, the researchers harnessed VHA data to conduct a separate analysis of 13,654 patients hospitalized with COVID-19 compared with 13,997 patients hospitalized with seasonal flu. All patients survived at least 30 days after hospital admission, and the analysis included six months of follow-up data.
The researchers confirmed that, despite being initially a respiratory virus, long COVID-19 can affect nearly every organ system in the body. Evaluating 379 diagnoses of diseases possibly related to COVID-19, 380 classes of medications prescribed and 62 laboratory tests administered, the researchers identified newly diagnosed major health issues that persisted in COVID-19 patients over at least six months and that affected nearly every organ and regulatory system in the body, including:
Respiratory system: persistent cough, shortness of breath and low oxygen levels in the blood.
Nervous system: stroke, headaches, memory problems and problems with senses of taste and smell.
Mental health: anxiety, depression, sleep problems and substance abuse.
Metabolism: new onset of diabetes, obesity and high cholesterol.
Cardiovascular system: acute coronary disease, heart failure, heart palpitations and irregular heart rhythms.
Gastrointestinal system: constipation, diarrhea and acid reflux.
Kidney: acute kidney injury and chronic kidney disease that can, in severe cases, require dialysis.
Coagulation regulation: blood clots in the legs and lungs.
Skin: rash and hair loss.
Musculoskeletal system: joint pain and muscle weakness.
General health: malaise, fatigue and anemia.
While no survivor suffered from all of these problems, many developed a cluster of several issues that have a significant impact on health and quality of life.
Among hospitalized patients, those who had COVID-19 fared considerably worse than those who had influenza, according to the analysis. COVID-19 survivors had a 50% increased risk of death compared with flu survivors, with about 29 excess deaths per 1,000 patients at six months. Survivors of COVID-19 also had a substantially higher risk of long-term medical problems.
“Compared with flu, COVID-19 showed remarkably higher burden of disease, both in the magnitude of risk and the breadth of organ system involvement,” Al-Aly said. “Long COVID-19 is more than a typical postviral syndrome. The size of the risk of disease and death and the extent of organ system involvement is far higher than what we see with other respiratory viruses, such as influenza.”
In addition, the researchers found that the health risks from surviving COVID-19 increased with the severity of disease, with hospitalized patients who required intensive care being at highest risk of long COVID-19 complications and death.
“Some of these problems may improve with time — for example, shortness of breath and cough may get better — and some problems may get worse,” Al-Aly added. “We will continue following these patients to help us understand the ongoing impacts of the virus beyond the first six months after infection. We’re only a little over a year into this pandemic, so there may be consequences of long COVID-19 that are not yet visible.”
In future analyses of these same datasets, Al-Aly and his colleagues also plan to look at whether patients fared differently based on age, race and gender to gain a deeper understanding of the risk of death in people with long COVID-19.
Photo Credit: Sara Moser
A new study from Washington University School of Medicine in St. Louis shows that even mild cases of COVID-19 increase the risk of death in the six months following diagnosis and that this risk increases with disease severity. The comprehensive study also catalogues the wide-ranging and long-term health problems often triggered by the infection, even among those not hospitalized.
American Association for Cancer Research meeting is premier venue for presenting cancer research results
Newswise — Atlantic Health System Cancer Care physicians are co-authors of five original studies presented at this year’s AACR Annual Meeting, held virtually April 10-15 and May 17-21. The AACR meeting is one of the world’s premier scientific gatherings of cancer specialists and researchers.
“Atlantic Health System Cancer Care is extremely proud of its role, helping to lead these studies alongside some of the world’s best-known cancer researchers,” said Eric Whitman, MD, medical director, Atlantic Health System Cancer Care and director of the Atlantic Melanoma Center. “Our physicians conduct the most innovative research and provide world-class care seldom found outside of major academic medical centers. These innovative clinical trials offer more treatment options for patients, both in our area and in some cases, around the world.”
Studies presented at the AACR meeting were co-authored by Dr. Whitman; Missak Haigentz, MD, chair of hematology/oncology at Morristown Medical Center, medical director of hematology/oncology for Atlantic Health System and principal investigator, Atlantic Health Cancer Consortium NCORP; and Angela Alistar, MD, medical director of GI medical oncology and the Breakthrough Treatment Center at Morristown Medical Center. See below for links to the study abstracts, which are now live, and brief descriptions of the studies:
CT008 - Lifileucel (LN-144), a cryopreserved autologous tumor infiltrating lymphocyte (TIL) therapy in patients with advanced (unresectable or metastatic) melanoma: durable duration of response at 28 month follow up
Dr. Whitman; presentation
Dr. Whitman and colleagues presented 28-month follow-up data on the ongoing global phase 2, multicenter clinical trial of a new type of investigational cancer treatment known as lifileucel (LN-144) for advanced (Stage IV) melanoma. Lifileucel is a TIL (tumor infiltrating lymphocyte) therapy, in which the patient’s own immune system cells are removed from his/her tumor, treated with an immune booster and then infused back into the patient, along with a medication that stimulates the immune system. All participants in the study have metastatic melanoma that has progressed despite prior treatment with other therapies, including anti-PD-1 (checkpoint inhibitor) immunotherapy and BRAF/MEK inhibitor targeted therapy. At 28 months, lifileucel therapy has shown an overall response rate (ORR) of 36.4%. The study, which is ongoing, is sponsored by Iovance Biotherapeutics. Dr. Whitman and co-investigators presented earlier results at ASCO 2020. (NCT02360579CT201)
Early report of a phase I/II study of human placental hematopoietic stem cell derived natural killer cells (CYNK-001) for the treatment of adults with COVID-19 (NCT04365101)
Dr. Whitman; e-poster
Dr. Whitman and colleagues published interim phase 1 results from the first study to evaluate the safety and efficacy of CYNK-001 (Celularity, Inc.) to treat patients with SARS-CoV-2 (the virus that causes COVID-19). The investigational drug was previously only tested against various types of cancer. CYNK-001 is the only cryopreserved, allogeneic, off-the-shelf, natural killer (NK) cell therapy being developed from placental hematopoietic stem cells as a potential treatment option for various blood cancers, solid tumors, and infectious disease. NK cells are a unique class of immune cells, innately capable of targeting cancer cells and interacting with adaptive immunity (specialized immunity to certain disease causing agents). CYNK-001 has been shown to be toxic to various types of cancer cells and secretes immune-modulating cell-signaling proteins (cytokines) when they reach their target. Patients with moderate to severe COVID-19 not requiring intensive care or mechanical ventilation were enrolled in this study. Infusions of CYNK-001 were generally well tolerated, and three of four patients showed improvement in oxygenation, lung inflammation and medical imaging findings. Phase 1 is still ongoing. Once it is complete, a randomized, phase 2 clinical trial will test the efficacy of this therapy for SARS-CoV-2 against the best available therapy.
1671 - Evaluation of total PD-1 expression using multi-color flow cytometry in metastatic non-small Cell lung cancer patients treated with multi-neoantigen vector (ADXS-503) alone and in combination of pembrolizumab to assess T-cell & T-cell memory subset
Dr. Haigentz; e-poster
Dr. Haigentz and colleagues tested novel laboratory assays developed by Precision for Medicine to be used as biomarkers in Advaxis clinical trials of ADXS-503 alone and in combination with pembrolizumab (Keytruda) for metastatic non-small cell lung cancer. ADXS-503 is a new type of investigational cancer drug that targets “hotspot” mutations that commonly occur in specific cancer types. Pembrolizumab is an immunotherapy that targets the PD-1 pathway, which protects cancer cells from immune attack. It is commonly used to treat this type of lung cancer. The researchers used the lab assays to examine frozen blood cells from individuals treated with the two-drug combination and those treated with ADXS-503 alone, in order to detect freestanding PD-1 proteins and PD-1 proteins bound to pembrolizumab. The assays were effective at detecting PD-1 on various immune T cells. Dr. Haigentz and colleagues state that the assays will help in the evaluation of total PD-1 expression in T cells when PD-1 blocking immunotherapies are used. These results may also support the combination of ADXS-503 with PD-1 focused therapies that could lead to more effective cancer immunotherapies.
CT174 - Phase II study of avelumab and trastuzumab with FOLFOX chemotherapy in previously untreated HER2-amplified metastatic gastroesophageal adenocarcinoma
Dr. Alistar; e-poster
In a phase 2 study, Dr. Alistar and colleagues tested a combination of avelumab, trastuzumab and FOLFOX with patients with previously untreated metastatic gastric and gastric cancer that overexpresses the HER2 gene. Avelumab (Bavencio) is a monoclonal antibody immunotherapy known as an anti-PD-L1 therapy or immune checkpoint inhibitor, while trastuzumab (Herceptin) is also a monoclonal antibody that targets HER2 receptors. FOLFOX is a standard combination of chemotherapeutic drugs. The researchers added avelumab to the standard trastuzumab and FOLFOX regimen for these types of cancer. Study investigators showed that this combination therapy generated anti-tumor activity and compared favorably to previous studies of trastuzumab and chemotherapy. Dr. Alistar and colleagues are excited about the potential for adding checkpoint inhibitors to the chemotherapy/trastuzumab combination for these types of advanced gastrointestinal cancers. Some patients in the study were still being treated at the time the research abstract was submitted to the AACR. Additional lab-based studies are now underway. Atlantic Health System Cancer Care is the only cancer program in New Jersey involved in this study. (NCT03783936)
CT177 - A multi-center phase 2a trial of the CXCR4 inhibitor motixafortide (BL-8040) (M) in combination with pembrolizumab (P) and chemotherapy (C), in patients with metastatic pancreatic adenocarcinoma (mPDAC)
Dr. Alistar; e-poster
To date, physicians have been unable to improve the health of people with metastatic pancreatic adenocarcinoma, the most common type of pancreatic cancer, with checkpoint inhibitor immunotherapy. Preclinical research has shown that the inhibition of the CXCR4 protein helps makes cancer cells more accessible to checkpoint inhibitors. In the second cohort of phase 2a COMBAT clinical trial, Dr. Alistar and colleagues tested BioLineRx Ltd.’s CXCR4 inhibitor BL-8040 in combination with checkpoint inhibitor pembrolizumab (Keytruda) and a type of chemotherapy against this Stage IV cancer. The study had encouraging results, with a confirmed objective response rate of 13.2%, 5.6 month median duration of benefit from the treatment, overall survival average of 6.5 months and progression free survival of four months. The investigators concluded that the therapeutic combination should be tested against this late-stage cancer in a randomized clinical trial. (NCT02826486)
For information on current Atlantic Health System Cancer Care clinical trials, go to: www.atlantichealth.org/research. To learn more about the 2021 AACR Annual Meeting, go to: https://www.aacr.org/meeting/aacr-annual-meeting-2021/.