News
Newswise — LOS ANGELES —
Cedars-Sinai has awarded nearly $700,000 to scientists developing new treatments and technologies — such as advanced genetic profiling and biomedical sensors that can be worn at home — to deliver individualized healthcare to patients.
The awards are part of a Cedars-Sinai campaign to transform its practice of medicine by harnessing advanced data on individuals’ specific genes, proteins, microbiome (bacterial communities) and other body chemistry, with the goal of tailoring therapies and medications for specific patients. Using this approach, patients one day might receive treatments as unique as their own fingerprints, based on tests that reveal their molecular makeups.
"Our goal is to drive the development of the newest technology and best research, coupled to the finest clinical practice, to rapidly deliver precise and personalized healthcare solutions," said Dermot McGovern, MD, PhD, FRCP(Lon), professor of Medicine and Biomedical Sciences and director of the campaign, known as Cedars-Sinai Precision Health.
The institutionwide effort is a partnership among scientists, clinicians and industry. The eight projects selected for funding deploy sophisticated technologies, including individualized genetic and protein profiling, high-speed culturing of bacteria, next-generation ultrasound and mobile biosensors. Using these high-tech tools, researchers are striving to forge new prevention strategies and treatments for a range of diseases and conditions, including heart disease, cancer and gastrointestinal disorders.
"Cedars-Sinai Precision Health is going to fundamentally change the way we practice medicine," said McGovern, the Joshua L. and Lisa Z. Greer Chair in Inflammatory Bowel Disease Genetics.
As one of the largest nonprofit academic medical centers in the U.S., Cedars-Sinai is in a strong position to advance this work. Its cadre of scientists, led by 350 principal investigators, is currently conducting about 1,500 bioscience studies.
While many institutions are pursuing precision healthcare strategies, Cedars-Sinai is especially suited to advance the promise of this medical revolution.
"Cedars-Sinai's flexible organizational structure, combined with its research talent and large-scale, high-quality healthcare delivery operation, make it well-qualified to achieve national prominence in the delivery of precise health solutions," said Shlomo Melmed, MD, executive vice president, Academic Affairs, and dean of the medical faculty at Cedars-Sinai. "With Cedars-Sinai Precision Health, we plan to lead the way to the newest frontier of medicine."
# # #
Newswise —
A new study by Johns Hopkins researchers suggests that a specialized area of the mosquito brain mixes tastes with smells to create unique and preferred flavors. The findings advance the possibility, they say, of identifying a substance that makes “human flavor” repulsive to the malaria-bearing species of the mosquitoes, so instead of feasting on us, they keep the disease to themselves, potentially saving an estimated 450,000 lives a year worldwide.
A report on the research appeared online on Oct. 3 in the journal Nature Communications. Malaria is an infectious parasite disease of humans and animals transmitted by the bite of the female Anopheles gambiae mosquito. In 2015, experts estimate it affected 214 million people, mostly in Africa, despite decades of mosquito eradication and control efforts. There is no malaria vaccine, and although the disease is curable in early stages, treatment is costly and difficult to deliver in places where it is endemic.
“All mosquitoes, including the one that transmits malaria, use their sense of smell to find a host for a blood meal. Our goal is to let the mosquitoes tell us what smells they find repulsive and use those to keep them from biting us,” says Christopher Potter, Ph.D., assistant professor of neuroscience at the Johns Hopkins University School of Medicine.
Because smell is essential to mosquito survival, each mosquito has three pairs of “noses” for sensing odors: two antennae, two maxillary palps and two labella. The maxillary palps are thick, fuzzy appendages that protrude from the lower region of the mosquito’s head, more or less parallel to its proboscis, the long, flexible sheath that keeps its “feeding needle” under wraps until needed. At the very tip of the proboscis are the labella, two small regions that contain both “gustatory” neurons that pick up tastes and olfactory neurons for recognizing odorants.
To better understand how An. gambiae mosquitoes that cause malaria receive and process olfactory information from so many sensory regions, Potter’s team wanted to see where olfactory neurons from those regions go to in the brain.
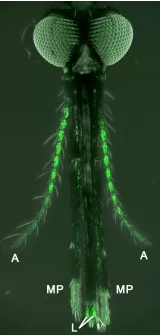
They used a powerful genetic technique — never before accomplished in mosquitoes, according to Potter — to make certain neurons “glow” green. The green glowing label was designed to appear specifically in neurons that receive complex odors through proteins called odorant receptors (ORs), since OR neurons are known to help distinguish humans from other warm-blooded animals in Aedes aegypti mosquitoes, which carry the Zika virus.
“This is the first time researchers managed to specifically target sensory neurons in mosquitoes. Previously, we had to use flies as a proxy for all insects, but now we can directly study the sense of smell in the insects that spread malaria,” says Olena Riabinina, Ph.D., the lead author of the study and a postdoctoral fellow now at the Imperial College London. “We were pleasantly surprised by how well our genetic technique worked and how easy it is now to see the smell-detecting neurons. The ease of identification will definitely simplify our task of studying these neurons in the future.”
As expected, Potter says, the OR neurons from the antennae and maxillary palps went to symmetrical areas of the brain called antennal lobes, just as they do in flies. But Potter was quite surprised when he saw that the OR neurons from the labella went to the so-called subesophageal zone, which, he says, had never before been associated with the sense of smell in any fly or insect; it had only been associated with the sense of taste.
“That finding suggests that perhaps mosquitoes don’t just like our smell, but also our flavor,” says Potter. “It’s likely that the odorants coming off our skin are picked up by the labella and influence the preferred taste of our skin, especially when the mosquito is looking for a place to bite.”
Potter says the finding potentially offers researchers one more way to repel mosquitoes. The antennae and maxillary palps are more specialized for picking up long-range signals, while the labella come into direct contact with our skin. In fact, Potter says, before injecting their needlelike proboscis, mosquitoes use the labella to probe about on a victim’s skin. “We don’t really know why they do that, but we suspect that they’re looking for sensory cues that hint at easy access to a blood vessel,” he says. “This suggests that a combination of repellants could keep mosquitoes from biting us in two ways. One could target the antennal neurons and reduce the likelihood that they come too close, while another could target the labellar neurons and make the mosquitoes turn away in disgust — before sucking our blood — if they got close enough to land on us.”
The two-part genetic system Potter devised to generate the glowing neurons will make it much easier for his and other laboratories to mix and match genetically altered mosquitoes to generate new traits, he says. His group has already created a strain of An. gambiae mosquitoes whose OR neurons glow green upon activation. Scientists can thus see which neurons light up in response to a specific smell.
“Using this method, we hope to find an odorant that is safe and pleasant-smelling for us but strongly repellant to mosquitoes at very low concentrations,” says Potter.
His group was also able to compare the brains of male and female mosquitoes. Since only females use their sense of smell to find humans and males feed only on nectar, it was previously thought that males had just a rudimentary sense of smell. The Potter group found instead that males have the same level of complexity in their brains to detect odors as females but have fewer olfactory neurons. “It appears that males might just have a scaled-down version of a female’s sense of smell. So they can still smell everything a female smells, just not as well,” Potter says.
His group plans to study other types of neurons to better understand how signals from the mosquitoes’ three types of olfactory receptors interact to influence their behavior. For example, why is lactic acid not attractive on its own but highly attractive when mixed with carbon dioxide?
“We’d like to figure out what regions and neurons in the brain lead to this combined effect,” says Potter. “If we can identify them, perhaps we could also stop them from working.”
Other authors of the report include Darya Task, Elizabeth Marr and Chun-Chieh Lin of the Johns Hopkins University School of Medicine; and Robert Alford and David O’ Brochta of the University of Maryland, College Park.
This work was supported by grants from the Johns Hopkins Medicine Discovery Fund, the Johns Hopkins Malaria Research Institute, and the National Institute of Allergy and Infectious Diseases (R01AI099060).
SEE ORIGINAL STUDY
Newswise —
Over the course of the Rio Olympics, 450,000 condoms were distributed around the athlete's village. This may be surprising considering the common view that abstinence from sexual activity can boost athletic performance.
These long-standing views have now been challenged by a recent analysis of current scientific evidence, published in the open-access journal Frontiers in Physiology.
"Abstaining from sexual activity before athletic competition is a controversial topic in the world of sport;" said Laura Stefani, an Assistant Professor of Sports Medicine at the University of Florence, Italy, and lead author of this review;"We show no robust scientific evidence to indicate that sexual activity has a negative effect upon athletic results."
The authors sifted through hundreds of studies with the potential to provide evidence, however big or small, on the impact of sexual activity upon sport performance. After setting a number of criteria to filter out the most reliable of these studies, only nine were included in the review.
One of these found that the strength of female former athletes did not differ if they had sex the night before. Another actually observed a beneficial effect on marathon runners' performance. While these small handful of studies provided some clues about the real effects of sex on sport performance, Dr. Stefani and her colleagues were disappointed with the research on this subject to date.
"We clearly show that this topic has not been well investigated and only anecdotal stories have been reported;" explained Dr. Stefani; "In fact, unless it takes place less than two hours before, the evidence actually suggests sexual activity may have a beneficial effect on sports performance."
The review also revealed that males were more frequently investigated than females, with no comparison of effects across genders. In addition, it highlights that cultural differences in attitudes towards sexual activity may influence how much or how little impact it may have. Dr. Stefani emphasizes other factors that have been ignored.
"No particular importance has been laid on the psychological or physical effects of sexual activity on sports performance, or upon the different kinds of sports."
This is an important point, given each sport's different mental and physical challenges.
This review demonstrates the need for proper scientific investigation into the impact of sexual activity on sport performance, clarifying any ethical, gender and sport differences.
The authors conclude that because the current evidence debunks the long-held abstinence theories, athletes should not feel guilty when engaging in their usual sexual activity up to the day before competition.
###
Newswise —
Atopic dermatitis, or eczema, is a common skin disorder that usually starts by 5 years of age, but virtually all of the studies that have defined the immune changes underlying eczema and are directing new treatment options have been done in adult skin. A study just published in the Journal of Allergy and Clinical Immunology characterizes immune changes for the first time in the skin of young children with eczema.
“Our findings offer new directions for targeted therapies for children with eczema,” says Amy Paller, MD, co-senior author and dermatologist at Ann & Robert H. Lurie Children’s Hospital of Chicago, as well as Chair of Dermatology and Professor of Pediatrics at Northwestern University Feinberg School of Medicine. “While some characteristics of eczema in children are the same as in adults, our study showed substantial differences that are important for understanding eczema in children and developing tailored treatments that maximize effectiveness and minimize potential side effects.”
Currently, there are no targeted therapies for affected children, who are usually treated with topical steroids and, in severe disease, with immunosuppressant drugs. This new study evaluated both affected and unaffected skin in children within the first 6 months of their eczema, and compared the immune profiles to those in skin from healthy children and also to the affected and unaffected skin of adults with eczema.
The study verified the central and early role of the immune pathway that drives allergy (Th2), which helps explain the association of eczema, asthma, and allergies in kids. In a previous study, Paller and her associates found evidence of activation of this Th2 immune pathway in the blood as well. This pathway is currently the focus of development of targeted therapy for moderate-to-severe eczema, suggesting that these same agents being tested in adults could also be useful in young children. The observed increase in the biomarker for itch (IL-31) in skin points to another useful therapeutic target for children.
In studies of blood samples from children with and without eczema during the first years of the life, the eczema group showed suppressed development of an immune pathway that handles infections (Th1). This observation may help to explain why children with more severe eczema have more widespread infections of herpes and molluscum viruses.
Several differences in the skin of children with eczema raise doubts about findings in adults that are now considered central to understanding eczema. Adult eczema skin is deficient in the naturally produced agents that fight staph infections and certain viruses, and this deficiency has been thought to be an important reason for the high risk of these infections in eczema at all ages. But the levels of these antimicrobial factors in the skin of young children with eczema are very high, suggesting that deficiency does not play a role in the frequent skin infections at early ages.
A characteristic trait of eczema at any age is a poor skin barrier, which leads to dryness and easier entry of triggers of the immune system, such as bacteria, irritating substances and allergens. Filaggrin, a key protein in skin barrier function, is deficient in adults. This has been attributed to both genetic deficiency and immune cell products that prevent production of filaggrin. The deficit of filaggrin has been blamed for the poor skin barrier in eczema. In contrast to adults, however, the skin of young children with eczema was found to have plenty of filaggrin, despite its poor barrier function and skin thickening that is comparable to skin of adults with eczema. This unexpected finding suggests that the filaggrin deficit may not be the driving force for barrier issues and that treatments targeting this protein might not be as beneficial for children with eczema as for adults.
A panel of findings was generated from the blood and skin samples of each participating child affected by eczema. “Our study is the first step toward personalized medicine for children with eczema,” says Paller. “We need to collect much more data to start matching targeted treatments to an individual’s specific disease characteristics.”
Research at Ann & Robert H. Lurie Children’s Hospital of Chicago is conducted through the Stanley Manne Children’s Research Institute. The Manne Research Institute is focused on improving child health, transforming pediatric medicine and ensuring healthier futures through the relentless pursuit of knowledge. Lurie Children’s is ranked as one of the nation’s top children’s hospitals in the U.S.News & World Report. It is the pediatric training ground for Northwestern University Feinberg School of Medicine. Last year, the hospital served more than 174,000 children from 50 states and 48 countries.
Newswise — ROCHESTER, Minn. —
After highlighting that more than half of American physicians are experiencing burnout, Mayo Clinic researchers now have identified some solutions that are being used to prevent or lessen burnout around the world. The findings show that some of the approaches being used are effective and making a difference. The article appears in the journal The Lancet.
The researchers identified more than 2,600 research articles that dealt with outcomes and approaches to physician burnout. They found 15 randomized clinical trials and 37 cohort studies that collectively included more than 3,600 physicians.
“We conducted an extensive search and compared the effectiveness of interventions across a range of burnout outcomes,” says lead author Colin West, M.D., Ph.D. “It’s clear that both individual strategies and structured organizational approaches are effective in achieving clinically meaningful reductions in burnout.”
Effective individual-focused strategies include mindfulness training, stress management training and small group sessions. Organizational changes that seem to work include limiting physician duty hours and a range of care delivery process changes in hospitals and clinics.
Mayo Clinic has been using some of these approaches with noticeable effects, including group interaction sessions in which the institution provides a designated lunch gathering monthly for breakout groups of physicians, so they can talk confidentially about their experiences with each other according to a structured curriculum.
The investigators say more research is needed as most published data come from observational studies and that validation of many of the solutions still is needed. In addition, the effect of combinations of interventions and their long-term benefits should be the focus of additional study.
Co-authors of the article are Liselotte Dyrbye, M.D., Tait Shanafelt, M.D. and Patricia Erwin, M.L.S. The research was supported by the Arnold P. Gold Foundation Research Institute and Mayo Clinic.
###
About Mayo ClinicMayo Clinic is a nonprofit organization committed to clinical practice, education and research, providing expert, whole-person care to everyone who needs healing. For more information, visit http://www.mayoclinic.org/about-mayo-clinic orhttp://newsnetwork.mayoclinic.org/.
Newswise — TALLAHASSEE, Fla. —
What can a Thai water bug teach us about our muscles, especially the heart?
A lot, says Professor of Biological Science Kenneth Taylor. New research by Taylor published today in Science Advances gives scientists better insight into how the heart muscle works and how sometimes it fails.
Taylor and his team used an electron microscope to capture the first three-dimensional image of a tiny filament, or strand, of an essential muscle that the palm-sized water bug Lethocerus indicus uses to fly. This filament is made of chains of a protein called myosin, which produce the power needed to contract muscles. This image shows for the first time the individual molecules in the filament in a relaxed state, which is necessary to re-extend muscles.
“After you contract your bicep to see if your muscles look like Arnold Schwarzenegger’s, you need this filament to assume its relaxed structure, so that after contraction your tricep muscle can re-extend your bicep,” Taylor said.
Scientists have long examined the flight muscles from Lethocerus indicus as a way to better understand how the human heart works. Both the insect’s flight muscle and a mammal’s heart beat rhythmically.
Mutations causing many inherited heart muscle diseases have been identified but are difficult to study in mammals because the heart is essential to life. However, these mutations can be studied in insects, specifically through this filament. Mutations that alter myosin function even slightly can have cumulative effects in a muscle contracting rhythmically.
While capturing a clear picture of this exact filament has been difficult, improved technology allowed Taylor and his fellow researchers to record an amazingly detailed image showing the precise filament structure.
All muscles have two types of filaments, actin and myosin. The chief difference between actin and myosin is that myosin has two parts, a molecular motor and a very long rod. The rods from many myosin molecules form the filament backbone. When a muscle contracts, molecular motors on the myosin filament grab and pull on the actin filaments causing the muscle to shorten. The packing of the myosin rods within the backbone must be strong enough to sustain this force.
With this new image, scientists can see how the molecular motors are arranged to prevent contact with the actin filament facilitating the muscle’s re-extension. At the same time they can see the tight packing of the myosin rods much “like a bamboo forest,” Taylor said.
“The image answers a whole lot of questions about myosin filaments that scientists have been wondering about for decades,” Taylor said.
The discovery is even more important because mutations in myosin can cause cardiomyopathy — a disease of the heart muscle. About one-third of myosin mutations that cause cardiomyopathies occur in the rod.“Many of these cardiomyopathy mutations may be understandable in terms of flawed muscle relaxation,” Taylor said.
This detailed image “shakes up the muscle field,” Taylor said. He hopes this breakthrough leads to novel treatments for cardiomyopathy in the future.
Now Taylor and his team will boost the resolution of these images so they can clearly see individual amino acids and accurately determine the key interactions between them.“We study insect flight muscle because it is a simpler route to understanding human disease,” Taylor said. “Ultimately, we must understand human disease from either human filaments or at least mammalian filaments.”
Other researchers contributing to the work are FSU molecular biophysics graduate student Zhongjun Hu and Research Associate Dianne Taylor, plus Duke University researchers Michael Reedy and Robert Edwards.
The research is funded by the National Institutes of Health and the American Heart Association.
###
Newswise — DALLAS –
When exposed to cold, clusters of cells within the body’s white fat become beige – a color change that reflects the creation of more energy-producing mitochondria, cellular components that enable cells to burn calories and give off heat. But since white fat cells have very few nerves, how do beige fat cells get the message that it’s cold outside?
In research that has implications for diabetes and other metabolic diseases, an international study based at UT Southwestern Medical Center found that the protein connexin 43 (Cx43) forms cell-to-cell communication channels on the surface of emerging beige fat cells that amplify the signals from those few nerve fibers. The channels act like conduits that speed signals across the gaps between clusters of cells – similar to the way a group email reaches several people at once.
The study, recently published in Cell Metabolism, also found that beige fat, unlike the better-known white and brown fat, has interesting anti-diabetic effects on blood sugar metabolism that seem independent of temperature regulation. Impaired glucose metabolism is a hallmark of diabetes.
“The data here show how white fat cells can make maximal use of their limited number of nerves to allow a single nerve fiber to spread the ‘message’ about cold temperatures amongst the connected cells,” explained Dr. Philipp Scherer, lead author of the study and Director of UT Southwestern’s Touchstone Center for Diabetes Research.
“To my knowledge, this is the first time that any fat’s thermal regulatory (warming) and metabolic effects on blood sugar have been observed to work independently. Our findings suggest that activating Cx43 may cause the formation of more beige fat and thus increase the anti-diabetic effects seen in this study,” Dr. Scherer added.
Fat, once considered merely a storage area for excess calories, is now appreciated as a dynamic tissue that comes in several forms with different functions that are still being identified. White fat is used mainly for energy storage. Brown fat, the classic heat-generating fat, helps regulate body temperature, especially in newborns. Some white fat cells are capable of transforming into a third kind of fat, beige. Both brown and beige fat get their color from increased mitochondria that are added in response to cold and other environmental stimuli, he said.
To study the metabolic effects of beige fat, the researchers compared mice with Cx43 that are able to make beige fat normally to mice unable to make Cx43, meaning their white fat seldom got the message to change to beige in response to cold. After three weeks in cold temperatures, the mice were returned to normal temperatures and analyzed for glucose (blood sugar) metabolism.
The mice that produced Cx43 showed greater improvement in glucose metabolism, Dr. Scherer said. Yet, both groups of animals were still able to regulate body temperature, apparently through their brown fat stores, he said.
“This study reaches two conclusions: First, Cx43 is necessary for the propagation of nerve signals that lead to beiging of white fat tissue. Second, beige fat may be more interesting from an anti-diabetic, metabolic standpoint – a finding with significant clinical relevance – than from a body temperature, warming standpoint,” explained Dr. Scherer, who holds the Gifford O. Touchstone, Jr. and Randolph G. Touchstone Distinguished Chair in Diabetes Research.
Study co-authors from the Touchstone Diabetes Center included lead author Dr. Yi Zhu, a postdoctoral fellow and employee of Eli Lilly and Co.; former graduate student Dr. Caroline Tao; postdoctoral researchers Dr. Mengle Shao and Dr. Shangang Zhao; Dr. Olga Gupta, Assistant Professor of Pediatrics and Internal Medicine and a Dedman Family Scholar in Clinical Care; and Dr. William Holland and Dr. Rana Gupta, both Assistant Professors of Internal Medicine. Additional UTSW contributors were Joshua Johnson, a student in UT Southwestern’s Medical Scientist Training Program; Dr. Tiemin Liu, Instructor of Internal Medicine; Dr. Joel Elmquist, Chief of the Division of Hypothalamic Research, Professor of Internal Medicine, Pharmacology and Psychiatry, and holder of the Maclin Family Distinguished Professorship in Medical Science, in Honor of Dr. Roy A. Brinkley, and the Carl H. Westcott Distinguished Chair in Medical Research; and Dr. Kevin Williams, Assistant Professor Internal Medicine in the Division of Hypothalamic Research.
Researchers at the Chinese Academy of Medical Sciences and Peking Union Medical College; The Ohio State University; Xi’an Jiaotong University; the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH); Albert Einstein College of Medicine; and Joslin Diabetes Center also contributed.
The research was supported by the NIH, the Cancer Prevention and Research Institute of Texas (CPRIT); a Lilly Innovation Fellowship Award; a China Scholarship Council Scholarship, and the American Heart Association.
About UT Southwestern Medical CenterUT Southwestern, one of the premier academic medical centers in the nation, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty includes many distinguished members, including six who have been awarded Nobel Prizes since 1985. The faculty of almost 2,800 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide medical care in about 80 specialties to more than 100,000 hospitalized patients and oversee approximately 2.2 million outpatient visits a year.
###
Newswise — (Chicago) –
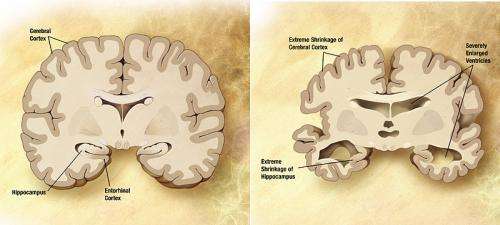
A recently-recognized pathologic protein in the brain may play a larger role in the development of clinical Alzheimer’s disease dementia than previously recognized, according to a study by researchers at Rush University Medical Center. The findings of the study of nearly 1,000 older adults were published in the Sept. 30 issue of the journal, Brain.
“This finding could help researchers to understand the cause of memory loss and lead to new ways to approach studying Alzheimer’s disease,” said Bryan James, PhD, study author and epidemiologist with the Rush Alzheimer’s Disease Center. “Our study found that when the main characteristic pathologies of Alzheimer’s disease, plaques and tangles, were mixed with a pathologic protein called TDP-43 in the brain, the combination was more likely to result in diagnosed Alzheimer’s dementia than plaques and tangles alone.”
The abnormal protein, TDP-43 (short for ‘hyperphosphorylated transactive response DNA-binding protein 43’), previously has been associated with frontal temporal dementia and amyotrophic lateral sclerosis (ALS, sometimes called Lou Gehrig’s disease). In recent years, TDP-43 also has been found in the brains of persons with other diseases, but most recently in Alzheimer’s disease.
Mixed pathologies increase Alzheimer’s risk
The hallmark pathologies of Alzheimer’s disease are the accumulation of the protein beta-amyloid (called plaques) and an abnormal form of the protein tau (called tangles). However, research from the Rush Alzheimer’s Disease Center and other groups has shown that the majority of persons with clinical Alzheimer’s dementia also develop other disease pathologies in their brains as well, such as small strokes or protein deposits called Lewy bodies.
This combination, called ‘mixed pathologies,’ increases the risk for developing diagnosed Alzheimer’s dementia above and beyond just having plaques and tangles in the brain.
“The clinical disease that we call ‘Alzheimer’s disease’ is looking more and more like the result of the accumulation of a number of disease processes in the brain of older persons,” James said. The majority of persons with diagnosed Alzheimer’s dementia actually have mixed pathologies in their brains — not just the plaques and tangles that are the known hallmarks of Alzheimer’s disease.
“In particular, mixed Alzheimer’s and TDP-43 pathologies appear to be an under-recognized yet common form of mixed pathologies that contributes to the development of clinical Alzheimer’s dementia,” James said. “This is one of the first studies to examine TDP-43 and Alzheimer’s disease in the context of mixed pathologies.”
TDP-43 found in two-thirds of those with Alzheimer’s dementia
The Brain paper built on previous research by examining whether TDP-43 was associated with an increased likelihood of a diagnosis of Alzheimer’s dementia in persons both with and without pathologic Alzheimer’s disease. The new study examined brain pathology, drawing on tissue samples from 946 deceased older men and women who had been enrolled in one of two cohort studies by the Rush Alzheimer’s Disease Center, the Rush Memory and Aging Project or the Religious Orders Study. Participants in both studies agree to donate their brains to research after their death.
TDP-43 was present in the brains of about half of the participants and in two-thirds of the brains of persons who had been diagnosed with Alzheimer’s dementia while alive. More than a third of the participants had mixed Alzheimer’s (plaques and tangles) and TDP-43 pathologies in their brain. Mixed Alzheimer’s and TDP-43 pathologies were associated with a higher likelihood of diagnosed Alzheimer’s dementia at death than plaques and tangles alone.
“These data are exciting, because an improved understanding of the TDP-43 protein has potential to guide alternative treatment strategies for Alzheimer’s disease,” James said.
Newswise —
Highly caffeinated energy drinks (EDs) have been of concern to the public-health community for almost a decade. Many young people consume EDs with alcohol to decrease alcohol’s sedative effects and stay awake longer, enabling them to drink more alcohol. Adding to the growing body of research linking ED consumption with risk-taking and alcohol-related problems, this study examined its relationship with drunk driving. Importantly, the researchers differentiated between the different ways in which EDs are consumed: exclusively with alcohol, exclusively without alcohol, or both with and without alcohol depending on the occasion.
Researchers looked at data from a longitudinal study of college students assessed annually via personal interviews. In year six, 1,000 participants (550 females, 450 males) self-reported their past-year frequency of drunk driving, ED consumption patterns, alcohol use, and other caffeine consumption. The researchers’ statistical model accounted for several background risk factors for drunk driving in order to isolate whether ED consumption might explain any unique variance in drunk driving behavior.
Results indicated that ED consumption was present in 57 percent of students: 9 percent drank EDs exclusively with alcohol, 16 percent drank EDs exclusively without alcohol, and 32 percent drank EDs both with and without alcohol depending on the occasion. More frequent ED consumption was associated with more frequent drunk driving through two distinct pathways. First, echoing prior research, consuming EDs with alcohol was associated with heavier alcohol drinking and, thereby, with more frequent drunk driving. A second separate path was unexpected—namely, consuming EDs without alcohol contributed additional risk for drunk driving, regardless of alcohol drinking patterns. The second path suggests that mechanisms other than the promotion of heavy drinking by EDs are involved in promoting drunk driving. The authors encourage parents, clinicians, and college administrators to regard any style of ED consumption, whether with or without alcohol, as a warning sign that students might be at high risk for alcohol-related consequences such as drunk driving. The authors also reiterate earlier calls to caution students against consuming EDs with alcohol.
SEE ORIGINAL STUDY
Newswise — MINNEAPOLIS/ST. PAUL —
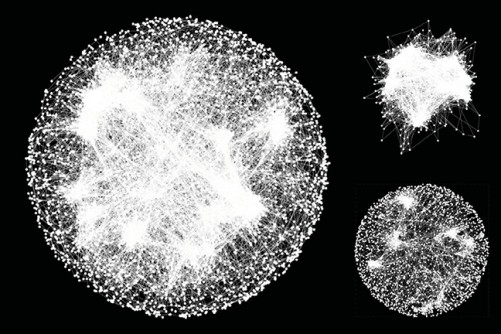
A global genetic interaction map is revolutionizing how genes are being studied. A new study, involving University of Minnesota researchers, is no longer looking at genes as loners, but instead as a social network of the body, interacting in groups. The new approach may ultimately change our understanding of the genetic roots of diseases.
The map will help scientists predict how genes function in order to understand, and thwart, the culprits behind diseases, with a potential for developing finely-tuned therapies. The research findings were published today in the journal Science.
Prior studies of yeast cells have shown only a fraction of genes, one out of five, was essential for a cell’s survival. This discovery was made by an international consortium of scientists over a decade ago where they targeted each of the yeast cell’s 6,000 genes for deletion. Recent advances in gene editing technology has allowed scientists to tackle the same question in human cells, resulting in the same answer—only a fraction of genes are essential to cell life.
Building on this research, University of Minnesota computer science and engineering Associate Professor Chad Myers, along with Professors Brenda Andrews and Charles Boone from the University of Toronto’s Donnelly Centre, have created the first complete genetic interaction network of a yeast cell, one that begins to explain how thousands of genes coordinate with one another to orchestrate cellular life.
“Even though many common diseases are thought to be caused by many different loci in the genome, we don't really understand the basic principles for how multiple genes combine to have effects,” Myers said. “Our comprehensive study of double mutant combinations in yeast establishes a set of first principles that we expect to apply in many different species, including humans.”
Biking without brakes
These findings suggest that most genes within our genomes are “buffered” to protect the cell from mutations and environmental stresses. Cells contain backup systems to ensure the essential functions of life keep working properly, even when one part is damaged. To address this buffering property, scientists had to ask if cells can survive upon losing more than one gene at a time, and they had to test millions of gene pairs.
Andrews, Boone, and Myers led the pioneering work in yeast cells by studying cell survival in the context of double mutants. To do so, they automated yeast genetic analysis, and they used robots to construct and examine almost all of the 18 million pairwise double mutant combinations.
The global genetic interaction map catalogues the pairs of genes that provide back up for one another—if the gene function of one is lost, the other gene in the genome fills its role.
Consider a bicycle analogy: a wheel is akin to an essential gene — a part without which it would be impossible to ride. Front brakes? That depends, because you could ride just fine as long as the back brakes are working. But what if you were to lose both sets of brakes? Without back brakes, the front brakes become essential, and vice versa.
Geneticists would call the relationship between front and back brakes as “synthetic lethal," meaning that losing both, but neither by themselves, spells doom. Synthetic lethal double mutant gene pairs are relatively rare, but when they are found, they reveal important information on gene pairs that work together to control essential functions.
Guilt by association
What’s more, the global map shows that synthetic lethal gene pairs are more likely to control the same biological process in the cell. This way, scientists are able to predict what a gene actually does in the cell simply based on its genetic interaction patterns, a process referred to as “guilt by association”.
If most genes in the human genome have one or more backup genes, then instead of searching for single genes underlying diseases, researchers now must look for gene pairs. This poses a huge challenge because they must somehow examine on the order of 200 million (!) possible gene pairs in the human genome that are associated with a disease.
Fortunately, with the know-how from the yeast map, researchers can now begin to map genetic interactions in human cells, and even expand it to a number of different cell types.
“Technology to manipulate human genomes on a large scale exists now,” Myers said. “Our work in yeast provides a blueprint for how we can learn about the human genome through systematic manipulation in cell lines.”
The concept of synthetic lethality is already changing cancer treatment because of its potential to identify drug targets that exist only in tumor cells. Cancer cells differ from normal cells in that they have scrambled genomes, littered with mutations. If scientists could find the highly vulnerable back-up genes in cancer, they could target specific drugs at them to destroy only the cells that are sick, leaving the healthy ones untouched.
This work was primarily supported by the National Institutes of Health, Canadian Institutes of Health Research, RIKEN Strategic Programs for R&D, Japan Society for the Promotion of Science Kakeni Grants, and the National Science Foundation.