The Valley Hospital in Ridgewood, NJ, Selected as 1 of 10 Hospitals to Participate
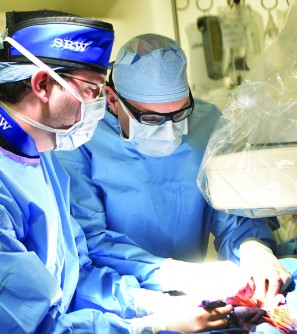
Newswise — The Valley Heart and Vascular Institute in Ridgewood, NJ, has been selected as 1 of just 10 hospitals – and the only hospital in New Jersey – to participate in a nationwide study investigating a minimally invasive aortic valve replacement procedure for individuals with aortic stenosis.
The first-of-its-kind clinical trial, led by Medstar Washington Hospital Center in Washington, D.C., aims to evaluate the use of transcatheter aortic valve replacement (TAVR), a minimally invasive procedure, in aortic stenosis patients who are at low risk for conventional open-heart surgery. TAVR is not yet approved by the FDA for use in this population. TAVR has already been established as a treatment option for intermediate- and high-risk patients. The trial is now open and will be enrolling 200 patients over a 24-month period. It will be non-randomized, meaning individuals who qualify will be selected to undergo the TAVR procedure instead of open-heart surgery.
"TAVR has already been shown to extend lives and improve the quality of life for intermediate- and high-risk patients who just a few years ago may not have had an alternative to open-heart surgery,” said John Goncalves, M.D., Director of Cardiac Surgery at The Valley Hospital and Surgical Director of Valley’s TAVR Program. “This is an exciting study to be a part of because it may potentially prove the effectiveness of TAVR for low-risk patients and open the door for others to qualify for this minimally invasive procedure in the future.”
TAVR is a procedure used to treat aortic stenosis, a life-threatening condition that occurs when blood flow is obstructed due to the narrowing of the aortic valve, one of the valves in the heart. Using TAVR, the heart team can replace a diseased aortic heart valve in a less-invasive manner than with open-heart surgery. The procedure requires only a small incision in the groin, through which an expandable heart valve is placed into the body and up to the heart via a catheter-based delivery system. Studies have shown that patients achieve better outcomes when undergoing TAVR than with medication alone.
“We are honored to be recognized for our expertise and experience with the TAVR procedure,” said Dr. Goncalves. “Clinical trials like this are critical to our work providing the highest quality and most innovative care for our patients.”
Individuals interested in making an appointment for an evaluation to see if they are a candidate for this study are encouraged to contact Jaclyn Chomsky, D.N.P., Valve Coordinator at Valley, at 201-447-8378 or chomja@valleyhealth.com.

